Table of Contents
What are Laminar Flow Cabinets?
Laminar flow cabinets (LFCs), also known as laminar flow hoods, are specialized enclosures designed to create a sterile environment by controlling airflow. These cabinets are essential in Good Manufacturing Practice (GMP) environments, as they protect sensitive processes from contamination. By ensuring a continuous, unidirectional flow of filtered air, laminar flow cabinets maintain a clean workspace crucial for activities such as pharmaceutical production, biotechnology, and clinical research.
This article explores the different types of laminar flow cabinets, including vertical and horizontal laminar flow cabinets, and sheds light on the key components such as pre-filters, High-Efficiency Particulate Air (HEPA) filters, and UV lights that ensure their optimal performance.
Principles of Functioning
The operational principle of a laminar flow cabinet revolves around creating a unidirectional airflow that moves in a single, continuous direction, typically from top to bottom or back to front, depending on the cabinet’s configuration. This unidirectional airflow is crucial in minimizing turbulence and ensuring that any particulates within the work area are promptly swept away and filtered out. The HEPA filters play a pivotal role in this process, capturing particles and microorganisms from the incoming air and ensuring that the environment within the cabinet remains virtually free of contaminants.
Air Filtration
The air filtration process in laminar flow cabinets is critical for achieving the desired cleanliness levels in GMP environments. These cabinets utilize High-Efficiency Particulate Air (HEPA) filters to remove 99.9 percent of microparticles from the air, including microorganisms and dust particles.
The air is passed through these filters in a unidirectional flow, either horizontally or vertically, depending on the cabinet’s design, ensuring that only clean, filtered air comes into contact with the work surface and the products being handled. This process is vital in maintaining a contamination-free environment, especially in the manufacturing of injectable drugs where any microbial contamination could compromise patient safety.
Maintaining Laminar Flow
Maintaining a homogenous air speed at the working position is essential for the effective operation of laminar flow cabinets. The cabinets are designed to provide a laminar or unidirectional air flow, which minimizes turbulence and prevents the introduction of contaminated air into the critical zone. This is achieved by directing the filtered air downward in a straight path or horizontally across the work surface, depending on the cabinet’s configuration.
The structure of the cleanroom, including the placement of laminar air flow hoods and cleanroom architecture, plays a significant role in lessening turbulence and maintaining the integrity of the laminar flow.
RELATED ARTICLE: Cleanroom Qualification and Validation in GMP
Ensuring Sterility
Ensuring sterility within laminar flow cabinets involves several critical practices, including minimizing the exposure of open containers, avoiding aseptic manipulations over an open system, and controlling movements within and adjacent to the critical space.
Personnel are required to wear sterile gloves and ensure that only their hands and wrists are in the laminar air flow to reduce movement in the critical zone. Additionally, materials should not be stored in the laminar flow, and any required materials should be decontaminated at the time of use. The cabinets themselves must be certified and have a visible calibration label for verification before use.
Regular monitoring through methods such as settle plates, volumetric air, and surface sampling is also essential for ensuring the sterility of the environment and the safety of the products being manufactured.
Importance of Laminar Flow Cabinets in Controlled Environments
In environments where the slightest contamination can lead to compromised results or product loss, such as in pharmaceutical manufacturing, microbiological research, or semiconductor production, laminar flow cabinets are indispensable. They provide the necessary sterile conditions for handling sensitive materials, thus playing a crucial role in maintaining quality and safety standards.
The ability to achieve ISO class 5 conditions within these cabinets underscores their importance in creating ultra-clean environments for critical processes in order to meet the stringent regulatory requirements of various industries, as well as maintaining high cleanliness and contamination control standards.
Regular maintenance and validation of laminar flow cabinets are crucial to ensure their optimal performance and compliance with GMP standards.
Applications in GMP Environments
Laminar flow cabinets are integral to various GMP environments, including:
- Pharmaceutical Manufacturing: Ensuring sterility in drug formulation and packaging.
Laminar flow cabinets minimize the risk of cross-contamination, a critical factor in the production of injectables and other sterile pharmaceutical products. The controlled environment provided by laminar flow cabinets is essential for the preparation of sterile drug compounds, IV admixture preparation, and other pharmaceutical procedures, ensuring product safety and compliance with regulatory standards.
The benefits of laminar flow cabinets in GMP regulated environments extend beyond maintaining sterility. They also contribute to the efficiency of processes by providing a controlled environment that minimizes the need for product rejections and rework due to contamination.
- Biotechnology: Protecting cell cultures and sensitive biological processes.
In biotechnology labs, laminar flow cabinets facilitate the culturing of microorganisms and handling of sensitive samples under sterile conditions. Their application in microbiology and biotechnology research is critical for preventing contamination during sensitive experiments and sample preparation. This ensures the reliability of research outcomes and the safety of biological products.
Regulatory Requirements for Laminar Flow Cabinets
LFCs play a crucial role in maintaining sterile environments in pharmaceutical manufacturing. Various regulatory bodies have established stringent guidelines to ensure that LFCs meet the necessary standards for preventing contamination and ensuring product quality. Here’s an overview of the regulatory requirements set forth by different organizations:
European Medicines Agency (EMA) and EudraLex
- Cleanroom and Clean Air Device Requirements: Laminar flow cabinets must provide an ISO Class 5 environment at the point of use to prevent contamination and ensure product quality.
- Qualification and Validation: LFCs must undergo rigorous qualification and validation processes, including installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ).
- Monitoring and Maintenance: Continuous monitoring of particle counts and air quality is required. Regular maintenance and filter changes are mandatory to maintain compliance.
Annex 1 of the EU GMP Guidelines (EudraLex Volume 4)
- Manufacture of Sterile Medicinal Products: This Annex specifically addresses the requirements for manufacturing sterile products, emphasizing the importance of cleanroom and clean air device standards.
World Health Organization (WHO)
- Air Handling Systems: LFCs should ensure unidirectional airflow and maintain ISO Class 5 conditions as per the WHO Technical Report Series, No. 961, Annex 6.
- Qualification: Similar to EMA, WHO requires LFCs to undergo IQ, OQ, and PQ to ensure they meet the necessary standards.
- Monitoring: Regular environmental monitoring for viable and non-viable particles is essential to maintain the sterility of the manufacturing environment.
United States Pharmacopeia (USP) <797> Pharmaceutical Compounding – Sterile Preparations
- Clean Air Equipment: LFCs must provide ISO Class 5 or better conditions.
- Placement and Use: LFCs should be placed in an ISO Class 7 buffer area or cleaner. When used for compounding sterile preparations, they must be properly located and maintained to ensure sterility.
- Certification and Maintenance: LFCs must be certified initially and recertified every six months or whenever relocated. Regular maintenance, including HEPA filter integrity testing, is required to ensure continuous compliance.
U.S. Food and Drug Administration (FDA)
- Clean Air Devices: LFCs must ensure unidirectional airflow and provide ISO Class 5 conditions to prevent contamination.
- Validation and Monitoring: Detailed validation and routine environmental monitoring protocols must be in place to ensure the effectiveness of LFCs.
- Operational Requirements: Proper design, operation, and maintenance of LFCs are critical to avoid contamination and ensure product sterility. Compliance with FDA guidance ensures that LFCs function correctly within the manufacturing environment.
Types of Laminar Flow Cabinets
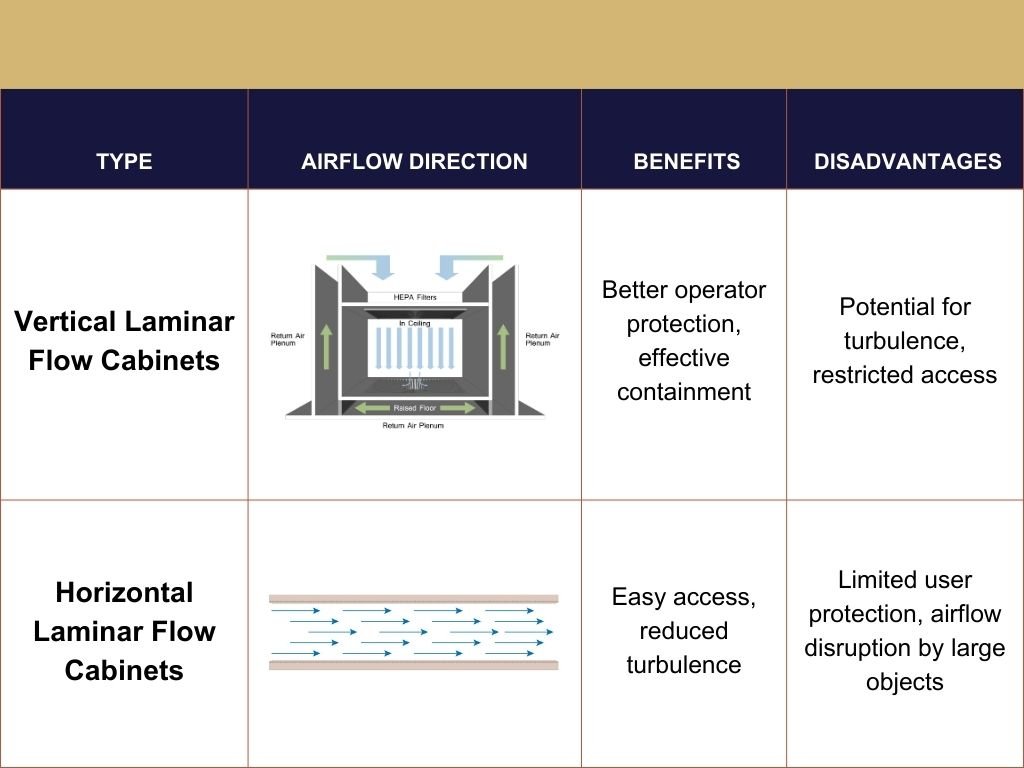
Laminar flow cabinets are available in two primary configurations: horizontal and vertical. Each type offers distinct advantages tailored to specific applications. Horizontal laminar flow cabinets direct the filtered air horizontally across the work surface, providing an unobstructed view and easy access to the workspace. This setup is particularly beneficial for tasks requiring precision and minimal physical strain.
Conversely, vertical laminar flow cabinets direct the air downward, from the top of the cabinet towards the work surface, offering a deeper work area and potentially better protection for the user from exposure to harmful substances.
Vertical Laminar Flow Cabinets
Vertical Laminar Flow Cabinets are characterized by their airflow direction, where filtered air is directed vertically downwards onto the work surface. This design facilitates the movement of air out of the working area through holes at the base or via a fixed opening, ensuring a contaminant-free environment. Known also as ‘Downflow Cabinets,’ these units are particularly advantageous for providing greater operator protection against harmful substances, thanks to the downward flow of air which minimizes direct contact with contaminants.
Vertical configurations are favored in scenarios where floor space is limited, as the fans and filters are positioned at the top of the unit, allowing these cabinets to be installed on standard laboratory benches. This setup not only reduces cross-contamination risks but also offers easier access for HEPA filter maintenance and replacement. However, it’s important to note that the vertical airflow might disturb smaller samples or open solutions due to direct air impact on the workbench.
Pros and Cons
Horizontal Laminar Flow Cabinets
Horizontal Laminar Flow Cabinets, discharge particle-free air horizontally across the work zone. The air, filtered through HEPA filters, enters from above and then changes direction to move horizontally, creating a barrier that prevents airborne contaminants from entering the work area. This configuration is beneficial for tasks requiring precision, as it provides an unobstructed view and easy access to the workspace.
These cabinets are designed with filters fixed to the back wall, pulling air from the back through the filter and across the length of the hood. This design minimizes turbulence and ensures a low risk of sample contamination by the user, making it ideal for handling sensitive experiments. However, it’s worth noting that large or bulky equipment can disrupt the laminar flow, potentially reducing the effectiveness of the hood.
Pros and Cons
Key Components and Design of Laminar Flow Cabinets
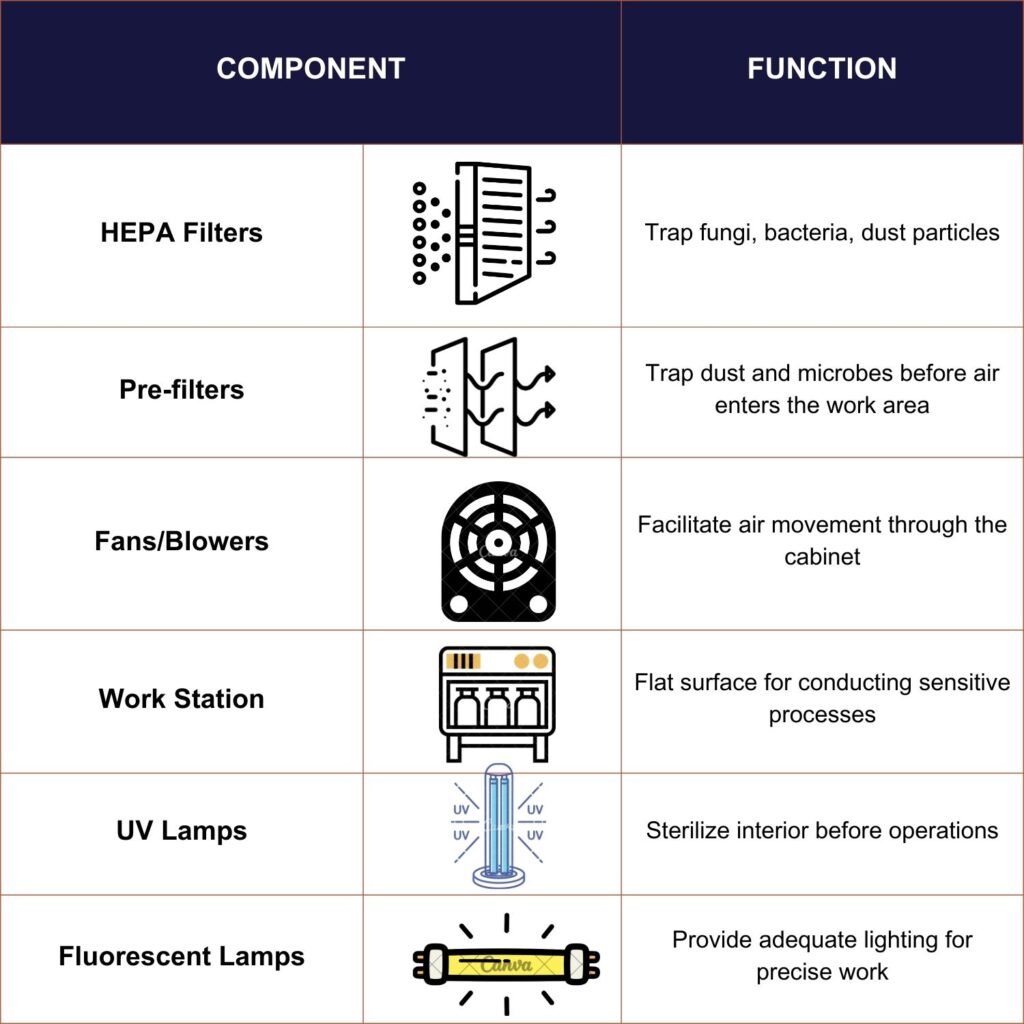
The core design of a laminar flow cabinet includes a filtration system that projects air through a HEPA filter, expelling it across a work surface in a laminar or unidirectional air stream. This design principle is fundamental in achieving the desired particle-free environment. The cabinets are predominantly constructed from stainless steel, chosen for its durability and ease of cleaning, and are meticulously designed without gaps or joints where contaminants might accumulate.
HEPA Filters
The most critical component of a laminar flow cabinet is the High-Efficiency Particulate Air (HEPA) filter. This filter is responsible for trapping fungi, bacteria, and other dust particles as air passes through it, thereby ensuring a sterile condition within the cabinet. The HEPA filter’s effectiveness is crucial for reducing the chances of contamination and maintaining the integrity of the work conducted within the cabinet.
Related Article: Types of HEPA Filters Used in Pharmaceutical Industry
Filter Pads / Pre-Filters
A filter pad or pre-filter is positioned at the top of the cabinet, playing a crucial role in trapping dust particles and some microbes before they enter the working environment. This component is essential for maintaining the cleanliness of the air that flows into the cabinet.
Fans / Blowers
Below the filter pad, a fan or blower is installed to facilitate the movement of air within the cabinet. This fan ensures that air circulates effectively throughout the cabinet, passing over the work surface and through the HEPA filter to trap any remaining contaminants.
Work Station
The work station within a laminar flow cabinet typically consists of a flat, often stainless steel surface where sensitive processes such as culture plating and sample preparation occur. This design minimizes the risk of rust and allows for easy cleaning and maintenance.
UV Lamps
Some laminar flow cabinets are equipped with a UV germicidal lamp to sterilize the interior and contents before operations begin. This lamp should be activated 15 minutes before use to ensure safety and effectiveness, and it must be turned off during operation to prevent exposure to UV radiation.
Fluorescent Lamps
To provide adequate lighting during operations, a fluorescent lamp is installed inside the cabinet. This lighting is crucial for precise work and visibility, ensuring that all procedures can be conducted with clarity and accuracy.
Do’s and Don’t’s for Laminar Flow Cabinets
Do’s:
- Regularly clean and maintain the cabinet according to manufacturer guidelines.
- Ensure proper training for all users to maintain a sterile environment.
- Perform routine checks on the HEPA filters and replace them as needed.
Don’t’s:
- Do not place large objects that obstruct airflow on the work surface.
- Avoid rapid movements that can disturb the laminar flow and cause turbulence.
- Never use the cabinet without verifying that the airflow and filtration systems are functioning correctly.
FAQ
Can Laminar Flow Cabinets Be Used for Both Sterile Compounding and Non-sterile Compounding in Pharmaceutical Production?
Laminar flow cabinets are specifically designed for maintaining sterility, making them ideal for sterile compounding processes such as the preparation of injectable drugs. For non-sterile compounding, different types of equipment may be more suitable depending on the level of contamination control required.
How Often Should the Hepa Filters in a Laminar Flow Cabinet Be Replaced to Ensure Compliance With GMP Standards?
The frequency of HEPA filter replacement depends on the usage and the specific requirements of your GMP environment. Generally, filters should be checked regularly and replaced at least once a year, or more frequently if performance tests indicate a drop in efficiency.
Are There Specific Guidelines for Decontaminating a Laminar Flow Cabinet After a Spillage of Biological Material?
Yes, in the event of a spillage, the area should be immediately decontaminated following the cabinet manufacturer’s protocols and your facility’s SOPs. This usually involves using an appropriate disinfectant, such as a 70% ethanol solution, and ensuring the cabinet runs for an additional period to clear any airborne contaminants.
How Do I Validate the Performance of a Laminar Flow Cabinet in My Pharmaceutical Lab?
Performance validation involves several steps, including airflow testing, particle count testing, and microbiological sampling. Regular certification by a qualified technician is required to ensure that the cabinet meets ISO Class 5 standards. Documentation of all validation procedures is essential for compliance with GMP regulations.
What Are the Best Practices for Introducing New Materials or Equipment Into the Laminar Flow Cabinet Without Compromising Sterility?
To maintain sterility, all materials and equipment should be thoroughly disinfected before being placed inside the cabinet. Introduce items slowly to avoid disrupting the laminar airflow. Additionally, minimize the frequency of introducing new items during critical operations to reduce contamination risk.
Conclusion
Laminar flow cabinets are vital in maintaining sterile environments in GMP settings, ensuring product quality and safety. By understanding the types, advantages, key components, and principles of functioning, you can make informed decisions about selecting and using laminar flow cabinets effectively in your facility. Proper maintenance and adherence to best practices will ensure the longevity and efficiency of these essential tools.















