Managing impurities in new drug products, including identification, quantification and defining specification limits, is of great concern for the pharmaceutical industry, given that most of these substances are characterized by toxic properties posing a harmful threat to human beings.
Defining specification limits for unknown, known and total impurities in a drug product can be quite a challenging process, depending on API synthesis, the manufacturing process, and product packaging and storage conditions. In this article, we explain how to set limits for known, unknown, and total impurities in new drug products, following the requirements in the ICH Q3B (R2) guideline.
Impurities in Drug Product Specifications
Specifications for new drug products should include a list of different types of impurities expected to occur during the manufacture of the commercial product and under recommended storage conditions.
The selection of degradation products in the specification should be based on the degradation profiles observed in safety and clinical development batches, as well as stability studies. The specification should include acceptance criteria for each specified degradation product, along with a general acceptance criterion for any unspecified degradation products.
Impurity Thresholds as Guidance for Specification Limits

When determining impurity specification limits for a new drug product, pharmaceutical manufacturers refer to the ICH Q3B R2 guideline to establish a framework for setting acceptable levels of known and specified impurities. By aligning their impurity specification limits with ICH’s established thresholds, manufacturers can guarantee that their products meet internationally recognized safety and quality standards.
Reporting Threshold
ICH defines the Reporting Threshold as the limit above which a degradation product shall be reported, meaning it represents the minimum concentration at which impurities must be identified and quantified. During the drug development and regulatory approval process, the reporting threshold plays a pivotal role in determining the level of impurity detection and reporting.
Setting an optimal reporting threshold is a delicate task as it involves a careful balance between the analytical capabilities of available testing methods and the potential impact of impurities on patient health. Setting up an excessively low reporting threshold can lead to unnecessary complications in regulatory filings and potentially hinder the development of life-saving medications, while a threshold that is set too high may overlook impurities that may pose a significant health risk.
Since we are mentioning analytical methods for the identification and quantification of impurities it is good to remember that during the validation of analytical methods, the quantitation limit should not be set higher than the reporting threshold. In such a scenario, we are setting up an analytical method that is not capable of adequately controlling the impurities that are required to be identified and quantified.
Setting up the Reporting Threshold Limits
As we previously discussed, the reporting threshold limits for impurities must strike a balance between the sensitivity of the analytical methods and the potential health risks the impurities pose. The Maximum Daily Dose (amount of drug substance administered per day) serves as a critical reference point for the reporting threshold, as it guides regulatory agencies and manufacturers in setting stringent limits for impurities. This approach ensures that even at the maximum dose, patient safety is never compromised.
- If the Maximum Daily Dose is ≤ 1 g, the Reporting Threshold for degradation products expressed as a percentage of the drug substance should be 0.1%
- If the Maximum Daily Dose is > 1 g, the Reporting Threshold for degradation products expressed as a percentage of the drug substance should be 0.05%
Exemptions for Reporting Threshold Limits
There are situations when the Reporting Threshold limits need to be defined with a different approach:
- Applicants who will set higher reporting thresholds will need to scientifically justify their proposition.
- For degradation products known to be unusually toxic or to produce unexpected pharmacological effects, reporting threshold limits are expected to be lower, and the quantitation and/or detection limit of the analytical procedure needs to correspond with the controlling levels set lower.
Identification Threshold
The identification threshold is defined by the ICH as a limit above which a degradation product should be identified. It represents the lowest concentration at which a specific impurity can be reliably detected and characterized using analytical techniques. It is recommended that specified identified degradation products be included in the list of degradation products alongside specified unidentified degradation products estimated to be present at levels greater than the identification threshold.
Using Identification Threshold to Set Up Limits for Unknown Impurities
Once a degradation product reaches the reporting threshold, it is obligatory to quantify and/or identify it. In situations where the structure of the degradation product is unknown, “How far can a degradation product be kept as an unknown impurity?” comes into question. As long as degradation products do not reach the identification threshold, they can be reported as unknown impurities.
Based on this, the identification threshold provides guidance for limiting unknown impurities. The Maximum Daily Dose is again the basis for setting different limits:
- If the Maximum Daily Dose is ≤ 1 mg, the Identification Threshold for degradation products expressed as a percentage of the drug substance should be 1.0% or 5 µg TDI (Total Daily Intake), whichever is lower
- If the Maximum Daily Dose is between 1 mg – 10 mg, the Identification Threshold for degradation products expressed as a percentage of the drug substance should be 0.5% or 20 µg TDI, whichever is lower
- If the Maximum Daily Dose is between > 10 mg – 2 g, the Identification Threshold for degradation products expressed as a percentage of the drug substance should be 0.2% or 2 mg TDI, whichever is lower
- If the Maximum Daily Dose is > 2 g, the Identification Threshold for degradation products expressed as a percentage of the drug substance should be 0.10%
*Example: If the Maximum Daily Dose for a drug substance is 0.3 mg (300 µg), the Identification Threshold should be 1.0% or 5 µg TDI, whichever is lower. Given the Maximum Daily Dose, 5 µg TDI is 1.67%. For this drug substance, the limit for unknown impurities (Identification Threshold) will be 1.0%.
What if the Degradation Product Concentration Is Greater Than the Identification Threshold?
For any unspecified degradation product, ICH Q3B R2 recommends including a general acceptance criteria of not more than the identification threshold. In cases where the degradation product concentration is greater than the identification threshold, new questions are raised. For more guidance on this section, we recommend checking ICH Q3B (R2)’s Attachment 3 (Figure 1) on “Identification and Qualification of degradation products in generic drug products”.
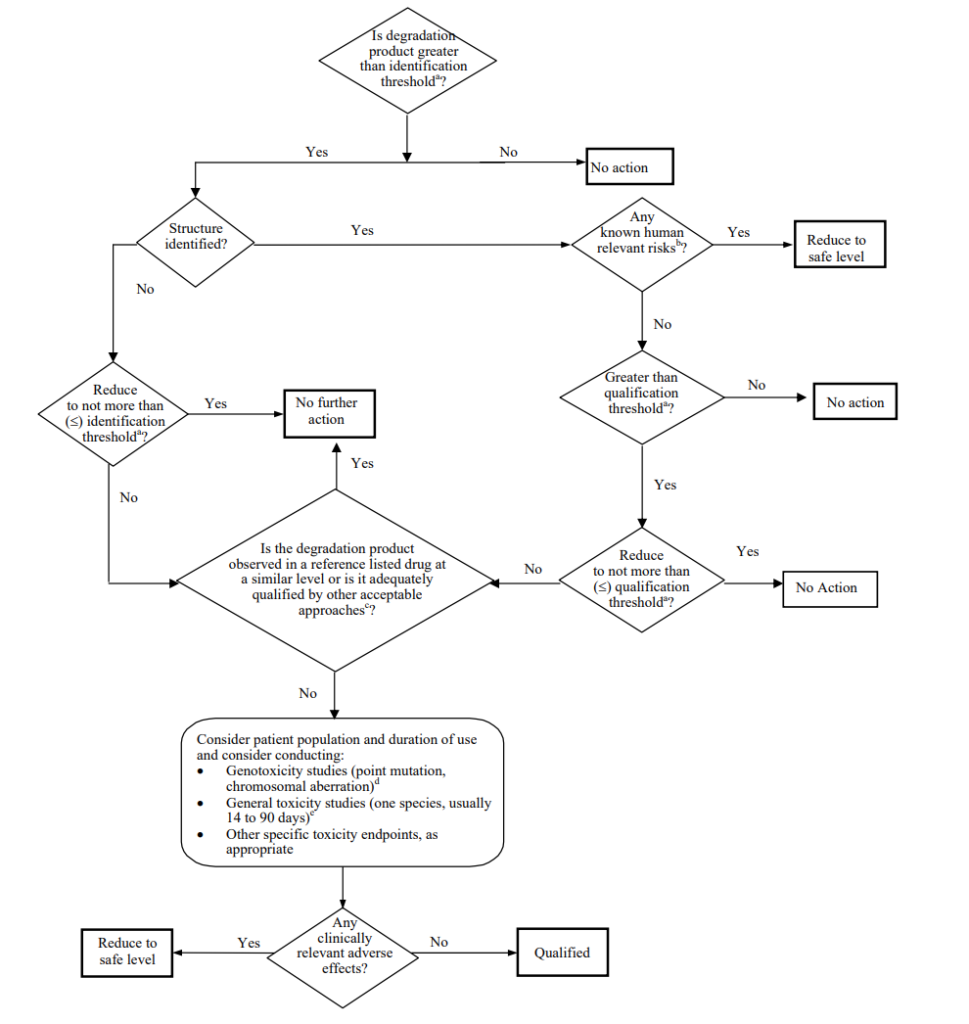
Qualification Threshold

The definition of Qualification Threshold presented by ICH is the limit above which a degradation product shall be qualified, meaning when a qualification threshold for a certain degradation product is exceeded, that degradation product should be qualified. Qualification of a degradation product involves the acquisition and assessment of data to determine the biological safety of a specific degradation product or degradation profile at the relevant level(s) with the help of toxicological studies.
When degradation product levels are being kept under the qualification threshold, there is no need to qualify the degradation product, meaning applicants are not obligated to conduct any type of safety study for this impurity.
Using Qualification Threshold to Set Up Limits for Known Impurities
When it comes to setting up limits for known impurities, this is where the Qualification Threshold of degradation products provides guidance based on the Maximum Daily Dose for a certain drug substance:
- If the Maximum Daily Dose is < 10 mg, the Qualification Threshold for degradation products expressed as a percentage of the drug substance should be 1.0% or 50 µg TDI, whichever is lower
- If the Maximum Daily Dose is between 10 mg – 100 mg, the Qualification Threshold for degradation products expressed as a percentage of the drug substance should be 0.5% or 200 µg TDI, whichever is lower
- If the Maximum Daily Dose is between > 100 mg – 2 g, the Qualification Threshold for degradation products expressed as a percentage of the drug substance should be 0.2% or 3 mg TDI, whichever is lower
- If the Maximum Daily Dose is > 2 g, the Qualification Threshold for degradation products expressed as a percentage of the drug substance should be 0.15%
*Example: If the Maximum Daily Dose for a drug substance is 6 mg (6000 µg), the Qualification Threshold should be 1.0% or 50 µg TDI whichever is lower. Given the Maximum Daily Dose, 50 µg TDI is 0.83%. For this drug substance, the limit for known impurities (Qualification Threshold) will be 0.83%.
Exemptions for Defining Limits for Known Impurities
In certain scenarios, drug products may have lower or higher limits for known impurities and setting up the limit with the help of the Qualification Threshold will not be applicable.
When to Set the Limit for Known Impurities Lower Than the Qualification Threshold
Limits for known impurities will probably be set lower than the Qualification Threshold:
- If the degradation product belongs to a class of impurities that are known to have an unusually potent toxic effect
*Example: A good example of this group of substances is the nitrosamine impurities. These impurities have raised significant concerns in the pharmaceutical industry due to their potential carcinogenicity and adverse health effects when consumed or administrated to humans
- If the impurity is known to be mutagenic, and guidance from toxicological concern can be found in ICH’s M7 guideline on “Assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk”.
- If the degradation product poses immunological or other types of clinical concerns
- If compendial limits are lower than the calculated Qualification Threshold
When to Set the Limit for Known Impurities Higher Than the Qualification Threshold
Limits for known impurities can be set higher than the Qualification Threshold:
- If compendial limits are higher than the calculated Qualification Threshold
- If data from toxicological studies suggests setting higher limits
- If data from comparative impurity analysis of a reference listed drug product show levels of a known impurity that exceed the Qualification Threshold
- If provided with data that a known impurity is a significant metabolite present in human and/or animal studies
Defining Limits for Total Impurities
When addressing limits for total impurities in a drug product, there are a few things worth mentioning:
- Limits for total impurities in a drug product can simply be defined by a summation of the limits for the individual specified impurities (identified and unidentified).
*Example:
In a drug product, there are two identified degradation products, and they are labelled as “Impurity A” and “Impurity B”. There is also one unidentified specified impurity with RRT = 0.8. Here below are the presented acceptance criteria for specified impurities: Impurity A = NMT 0.2%; Impurity B = NMT 0.6%; Unknown Impurity (RRT=0.8) = 0.3%;
Based on the limits for specified impurities, the limit for total impurities will be 1.1% (0.2% + 0.6% + 0.3%)
- When defining the limits for total impurities, the summation approach is not always applicable, as it is important not to exceed the thresholds that may compromise product’s potency
*Example:
The summation of the limits for all individual impurities in a product is 4.5%.
The assay limit for this drug product is 95.0% – 105.0%.
Verdict: In this example, the summation of limits for all specified impurities present in the drug product is not acceptable for defining the limits for total impurities.
FAQ: Defining Limits of Impurities in New Drug Products
For detailed information, you should check the ICH Q3B (R2) guideline and also refer to regulatory guidelines from agencies like the FDA, EMA, or specific pharmacopoeias, as well as scientific literature and resources provided by pharmaceutical industry associations.
Summation involves adding the individual known impurity levels to acceptable limits for unspecified impurities. This approach allows for flexibility in managing impurity profiles while maintaining overall impurity control.
Reporting Threshold limits may be adjusted if scientifically justified. They may be lower for exceptionally toxic impurities or higher if analytical methods are less sensitive.
If a degradation product concentration exceeds the Identification Threshold, it must be identified and characterized. There may be additional regulatory requirements for such impurities.
Yes, limits for known impurities can be adjusted in specific cases, considering factors such as toxicity, data from toxicological studies, or compendial limits.
Conclusion
The establishment of impurity limits in pharmaceutical products is a meticulous process guided by recognized standards and thresholds, such as those outlined in the ICH Q3B (R2) guideline. These limits, encompassing known, unknown, and total impurities, serve as critical parameters in ensuring product quality and patient safety throughout the drug development lifecycle.
It is essential that manufacturers exercise prudence and adherence to scientific diligence and GxP regulations when defining and applying these impurity limits, taking into account factors such as dosage, toxicity, and analytical capabilities. Moreover, aligning these limits with international regulatory expectations underscores the importance of maintaining rigorous control over impurities, ultimately contributing to the overall integrity of pharmaceutical products within the industry.














