Airflow visualization (AV), often referred to as smoke studies, is a technique used to make ambient air patterns visible for analysis and testing. These studies, which utilize smoke or fog for testing airflow, play a pivotal role in visualizing the airflow patterns within cleanrooms, isolators, and other controlled environments where aseptic processing takes place.
By harnessing the unique properties of smoke flow, specialists can effectively monitor and evaluate the performance of HEPA filters, laminar airflow systems, and the efficacy of aseptic technique, ensuring that the environment adheres to the rigorous standards of unidirectional airflow necessary for the prevention of contamination.
This article aims to provide a comprehensive step-by-step guide to planning, executing, and analyzing smoke studies, offering valuable insights into equipment selection, such as the appropriate smoke particle size for accurate airflow simulation, and detailing the processes involved in conducting a smoke test.
What Are Airflow Visualization Smoke Studies (AVS)?
Airflow visualization, often referred to as smoke studies, is a technique used to make the ambient air patterns visible for analysis and testing. This is typically achieved using devices like fog generators or smoke wands, which are essential tools in various settings, including HVAC systems, cleanrooms, and aseptic processing areas.
These devices help detect leaks, monitor turbulence in ventilation systems, and test the performance of HEPA filters. In critical environments, such as laboratories and pharmacies, airflow visualization ensures compliance with standards like USP <800> by testing airflow in biosafety cabinets and chemical fume hoods.
Importance in Cleanrooms and Aseptic Facilities
In the context of cleanrooms and barrier systems, airflow visualization studies are crucial for documenting actual airflow patterns. These studies are not only about compliance but also play a pivotal role in the operational quality and safety of manufacturing sterile medicinal products. The visualization aims to confirm the presence of unidirectional airflow, which is essential to sweep over and away from the product, thus maintaining aseptic conditions.
Understanding and controlling airflow is paramount in environments where sterile conditions are critical, such as in pharmaceutical manufacturing. Disruptions in airflow, caused by improper placement of objects or uncontrolled personnel movement, can lead to contamination risks. These risks make it essential to visualize and analyze airflow patterns meticulously. Conducting smoke studies allows for the identification and correction of eddy currents and deviant airflow patterns, which can be crucial for process optimization and training personnel.
Furthermore, regulatory frameworks like ISO 14644-3, EU GMP Annex 1, and US FDA guidance recommend airflow visualization studies to characterize and document airflow patterns. These studies help assess airflow direction and uniformity against design specifications and are integral in choosing locations for Environmental Monitoring as part of risk assessments in cleanrooms.
Types of Airflow Visualization

Airflow visualization studies ensure the proper functioning of cleanroom environments, particularly in maintaining the stringent standards required for Class A (ISO 5) classifications. These studies can be categorized into several types, each serving a specific purpose and conducted under different conditions. Understanding these types helps diagnose problems, validate designs, and ensure ongoing compliance with regulatory standards.
RELATED: GMP Cleanroom Classifications: Understand Class A, B, C and D
Investigative Airflow Visualization
Investigative airflow visualization studies are conducted to diagnose specific problems or investigate suspected issues within a cleanroom. These studies are typically initiated in response to anomalies observed in environmental monitoring data or contamination events.
Introducing smoke or other visualization agents into the environment allows these studies to trace airflow patterns around critical areas, equipment, or processes where issues are suspected. The primary goal is to identify the root causes of airflow problems, such as turbulence, dead spots, or incorrect flow patterns, and implement corrective actions to mitigate these issues.
Engineering Airflow Visualizations
Engineering airflow visualizations are performed during the design and construction phases of cleanroom facilities. These studies aim to validate that the airflow systems are functioning according to the design specifications before the cleanroom becomes operational.
Detailed smoke studies and computational fluid dynamics (CFD) simulations are commonly used to visualize and optimize airflow patterns. By conducting these studies early in the design process, potential airflow issues can be identified and addressed, ensuring that the cleanroom design supports the required cleanliness standards from the outset.
Static Airflow Visualization Studies
Static airflow visualization studies are conducted under at-rest conditions, meaning no personnel is present, to verify that the cleanroom or controlled environment meets specified airflow patterns and cleanliness levels when the room is in a static state.
These studies involve introducing a visualization agent, such as smoke, to observe the airflow patterns and identify any issues such as eddies or stagnant zones. Establishing baseline airflow patterns under these controlled conditions is essential for ensuring that the cleanroom can maintain its required standards before operational activities commence.
Dynamic In situ Air Pattern Analysis
Dynamic in situ air pattern analysis, also known as dynamic smoke studies, is performed under operational conditions. These studies assess how airflow patterns are affected by actual usage and activities within the cleanroom, including the movement of personnel and the operation of equipment.
By introducing smoke or other visualization agents while normal operations are being conducted, these studies provide a realistic view of how airflow behaves during real-world conditions. The goal is to ensure that the cleanroom maintains its cleanliness standards during active use and to identify and mitigate any disruptions in airflow caused by personnel movement or equipment operation.
Regulatory Requirements for Airflow Visualization
Airflow visualization is a critical aspect of maintaining and verifying the performance of cleanrooms, particularly those classified as Class A (ISO 5). It ensures that the airflow patterns meet the required standards to prevent contamination. Here are the specific requirements related to airflow visualization according to regulatory guidelines:
EU GMP Guideline, Annex 1
The EU GMP Guideline, Annex 1, provides essential standards for the manufacture of sterile medicinal products. Central to this guideline are requirements for cleanroom classification, monitoring, and operation to maintain a contamination-free environment.
Airflow Visualization Tests
- Objective: To demonstrate unidirectional airflow and identify any turbulence or stagnant areas that could compromise cleanroom performance.
- Method: This involves using a visible smoke or vapor source to trace airflow patterns. The smoke is introduced at various locations to fully visualize the dynamics within the cleanroom.
- Frequency: Conducted during initial qualification, after significant changes to the cleanroom or HVAC system, and periodically as part of routine requalification.
ISO 14644-3: Test Methods for Cleanrooms
ISO 14644-3 outlines the procedures and methods necessary to ensure the proper functioning of cleanroom environments.
Purpose: Confirm the direction, uniformity, and behavior of airflow in critical areas.
Techniques:
- Using smoke, fog, or other visualizing agents to observe airflow patterns.
- The visualization medium should be non-toxic and non-contaminating.
Procedure:
- Preparation: Ensure the cleanroom operates under normal conditions.
- Introduction of Visualization Agent: Introduce the agent at different points, including critical operations areas and around HEPA filters.
- Observation and Documentation: Record the behavior of the agent, looking for unidirectional flow and the absence of turbulence or stagnant zones. Document with video recordings, photographs, and a written report detailing observations and deviations.
Acceptance Criteria: Unidirectional airflow should be evident, with no recirculation zones that could lead to contamination. Any areas of concern should be addressed and rectified.
USP <797> Pharmaceutical Compounding – Sterile Preparations
USP <797> sets forth requirements to maintain clean and controlled environments for sterile compounding.
Purpose: Ensure adequate airflow within the compounding area, particularly within Primary Engineering Controls (PECs) like laminar airflow workbenches and biological safety cabinets, to protect the sterile environment.
Method: Conduct smoke studies to visualize airflow patterns within the PEC and the cleanroom. This helps identify any potential disruptions in the airflow that could lead to contamination.
Documentation: Document the results, including video or photographic evidence, to clearly show airflow patterns and highlight areas where the airflow is not unidirectional or is disrupted.
Common Airflow Visualization Tests and Methods
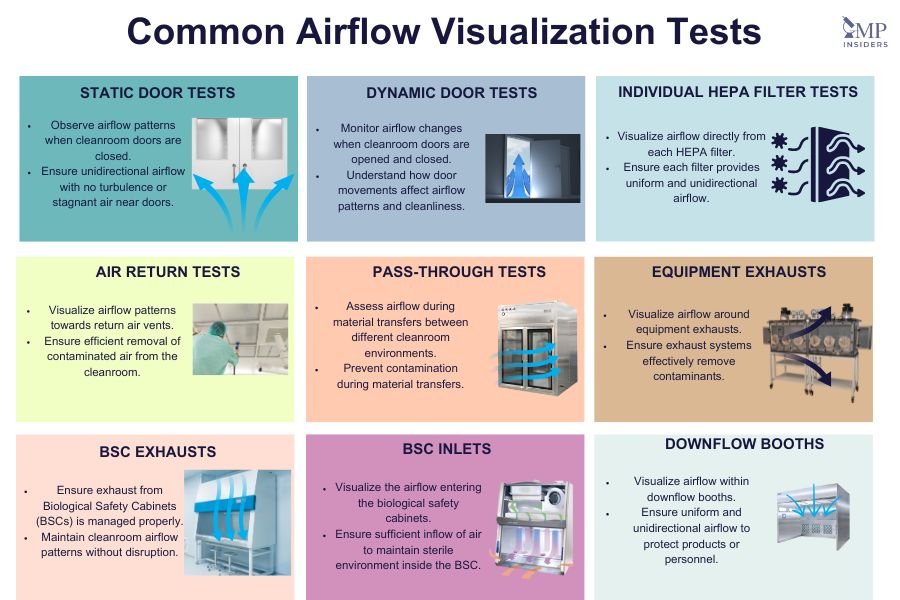
Airflow visualization tests are crucial for ensuring that cleanroom environments function correctly and meet regulatory standards. These tests help visualize and confirm the airflow patterns, detect potential issues, and validate the performance of various cleanroom components. Here are some common airflow visualization tests and methods used in cleanrooms:
Static Door Tests
Static door tests are performed to observe the airflow pattern when the cleanroom doors are closed. These tests help ensure that the airflow remains unidirectional and that there are no areas of turbulence or stagnant air near the doors. Smoke or other visualization agents are introduced near the doors to observe how the air behaves in this static state.
Dynamic Door Tests
Dynamic door tests are conducted with the cleanroom doors being opened and closed, simulating actual conditions during operations. These tests are essential for understanding how opening and closing doors affect the airflow patterns and the cleanroom’s ability to maintain its required cleanliness standards. The visualization agent is used to monitor airflow changes and identify any disruptions caused by the door movements.
Individual HEPA Filter Tests
Individual HEPA filter tests involve visualizing the airflow directly from each HEPA filter. The purpose is to ensure that each filter is functioning correctly, providing a uniform and unidirectional airflow. Smoke is introduced at the filter face to observe the airflow pattern and verify that there are no leaks or areas of inadequate airflow.
SEE ALSO: Types of HEPA Filters in Pharma Industry
Air Return Tests
Air return tests are performed to visualize the airflow patterns towards the return air vents. These tests help ensure that the contaminated air is efficiently removed from the cleanroom and that there are no areas where air is not being effectively exhausted. Visualization agents are introduced near the return vents to monitor the airflow.
Pass-Through Tests
Pass-through tests focus on areas where materials or products are transferred between different cleanroom environments or from lower to higher cleanliness levels. These tests ensure that the airflow patterns prevent contamination during the transfer process. Smoke is used to visualize the airflow in and around pass-through chambers to confirm that contaminants are not carried into cleaner areas.
Equipment Exhausts
Equipment exhaust tests involve visualizing the airflow patterns around the exhausts of various equipment used within the cleanroom. These tests ensure that the exhaust systems are effectively removing contaminants generated by the equipment. Visualization agents are introduced near the exhaust points to observe the airflow.
BSC Exhausts
BSC (Biological Safety Cabinet) exhaust tests are conducted to ensure that the exhaust from BSCs is properly managed and does not disrupt the cleanroom’s overall airflow patterns. Smoke is introduced at the BSC exhaust to visualize and confirm that the airflow is correctly directed and does not pose a contamination risk.
BSC Inlets
BSC inlet tests involve visualizing the airflow entering the biological safety cabinets. These tests ensure that the inflow of air is sufficient to maintain a sterile environment inside the BSC. Smoke is introduced at the BSC inlets to observe the airflow pattern and verify its effectiveness.
See Also: How to select the right BSC for your Lab?
Downflow Booths
Downflow booth tests are performed to visualize the airflow within downflow booths, which are used to protect products or personnel from contamination. These tests ensure that the downflow of air is uniform and unidirectional, effectively preventing contaminants from entering the protected area. Smoke is introduced within the booth to monitor the airflow pattern.
How to Plan a Smoke Study Plan
A well-structured plan is crucial for conducting effective smoke studies in cleanroom environments. The plan usually consists of:
Setting Objectives
The objectives of conducting smoke studies in aseptic processing facilities are to confirm that the airflow patterns are unidirectional and meet the required standards for both static and dynamic conditions. Static studies aim to document that air flows within the Class A/ISO 5 zones are unidirectional and cascade out to zones with lower cleanliness requirements.
Dynamic studies, on the other hand, focus on ensuring that airflow within the Grade A/ISO Class 5 filling lines remains unidirectional, sweeping down and away from sterile equipment surfaces, container/closure systems, and the product during operations.
Common Pitfall: Vague objectives can lead to unclear results.
Troubleshooting Tip: Ensure objectives are specific, measurable, and aligned with regulatory requirements.
Outlining Responsibilities
Responsibilities for the smoke studies should be clearly defined and include developing, implementing, reviewing, and approving the studies. This includes identifying the roles of individuals and teams, such as the isolator manufacturer, the owner of the process, and certifiers.
Each party has specific responsibilities, such as verifying the correct protocol for conducting the test, ensuring all materials for conducting the dynamic test are available, and confirming that those conducting the test are familiar with the protocol requirements.
Common Pitfall: Overlapping roles can cause confusion.
Troubleshooting Tip: Clearly document each role and communicate responsibilities to all team members.
Defining Acceptance Criteria
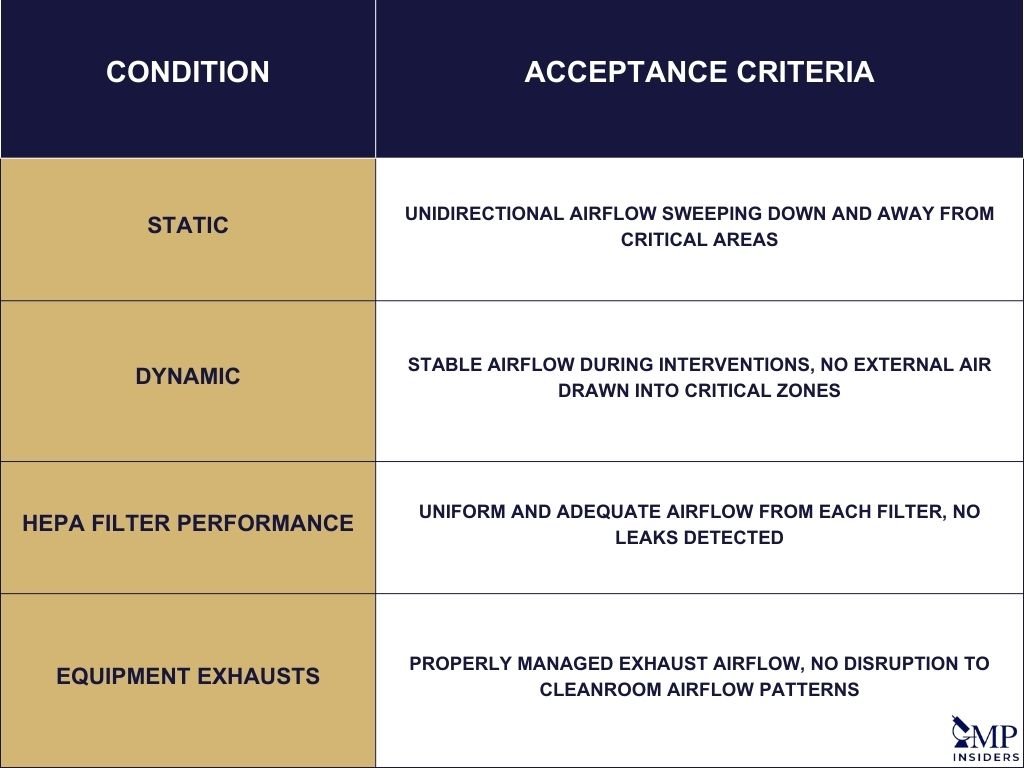
Acceptance criteria for smoke studies should be explicitly stated to ensure clarity and compliance. For static conditions, the criteria might include demonstrating that unidirectional airflow patterns sweep down and/or away from exposed products, product contact packaging components, and product contact surfaces.
In dynamic conditions, the criteria should confirm that the unidirectional airflow remains stable during interventions, with no disruption of first air to critical areas where sterile surfaces, materials, and products are exposed. Additionally, it should be verified that air from outside the critical Grade A/ISO Class 5 area does not get drawn into the critical zone during interventions.
To ensure comprehensive coverage, the smoke source should be positioned to allow sufficient smoke to enter the airflow over the operator and tools during interventions, thereby visualizing the impact of this activity on the airflow. It is crucial to generate enough smoke to merge with the airflow and visually display its direction, but not so much that it obscures the view of the operations.
Common Pitfall: Undefined or unrealistic criteria.
Troubleshooting Tip: Base criteria on regulatory standards and previous performance data.
Equipment Selection for Smoke Studies
Conducting smoke studies requires a selection of specific equipment to ensure accurate airflow visualization. Essential tools include various types of smoke generators, which produce the visualization medium, and video recording equipment to document airflow patterns. Additional tools, such as handheld anemometers and air return tests, help measure air velocity and assess the efficiency of the cleanroom’s airflow system.
Smoke Generators
Selecting the appropriate smoke generator is crucial for effective airflow visualization in smoke studies. The cleanroom fogger, which utilizes water for injection (WFI) or deionized water to generate smoke or fog, is highly recommended due to its ability to produce a high-purity fog through methods like mega sonic vaporization, steam, or dry ice.
Video Recording Equipment
Accurate documentation during smoke studies is essential. A video camera is indispensable for recording the smoke flow within the environment, allowing for later analysis of airflow patterns and identification of any issues. The personnel operating the camera must be well-trained, as filming in a cleanroom environment can be particularly challenging without the necessary skills or artistic inclination.
Additional Tools
Additional tools play a supportive yet vital role in conducting thorough and effective smoke studies. Handheld °C Point anemometers are invaluable for measuring air velocity with high accuracy. These devices are often Bluetooth-enabled, facilitating easy transmission of data to mobile devices for convenient analysis and record-keeping.
In settings where airflow through floor tiles needs assessment, °C Grate systems offer an advantageous solution by allowing air to flow freely, which helps in avoiding the inaccuracies common with flow-restricting methods.
For environments requiring detailed and precise airflow visualization, such as cleanrooms and aseptic processing areas, fog generators are used. These tools are crucial for revealing airflow patterns, identifying turbulence, testing filters, and detecting leaks. They must be selected based on their ability to produce a fog that closely mimics the properties of the airflow being studied, ensuring that the visualization is as accurate as possible.
Common Pitfall: Using inadequate or inappropriate equipment.
Troubleshooting Tip: Verify equipment specifications meet study requirements and perform calibration checks.
Conducting a Smoke Study
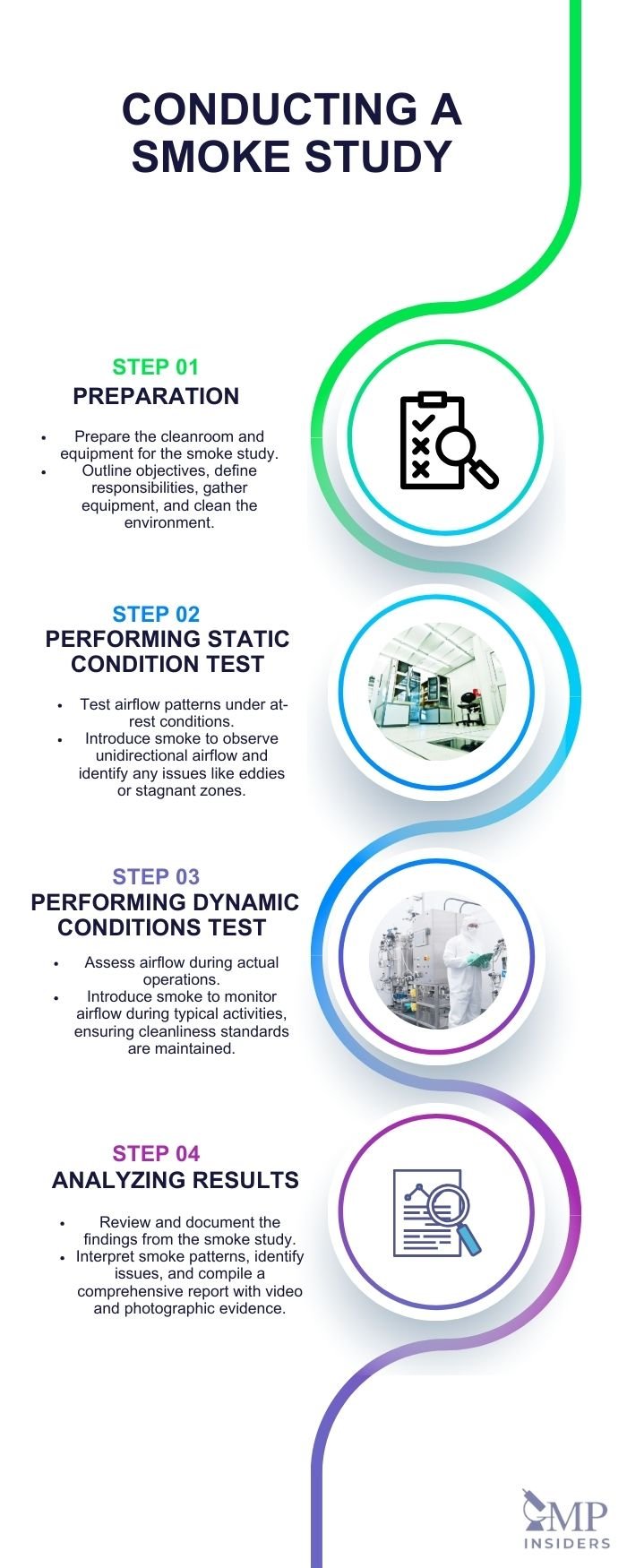
Proper execution of a smoke study is essential to validate cleanroom airflow patterns and ensure regulatory compliance. The Smoke Study consists of:
Preparation Steps
To initiate a successful smoke study, the protocol must clearly outline the objectives, responsibilities, and tools necessary for both static and dynamic conditions. The plan should include detailed floor diagrams of each filling room, showing the position of the operator during interventions, the smoke source, and camera positions to ensure comprehensive coverage.
It is essential to select the appropriate type of smoke generators, such as those using water for injection (WFI) combined with liquid nitrogen or dry ice, to generate sufficient smoke for clear visualization.
Common Pitfall: Inadequate cleaning and preparation can affect results.
Troubleshooting Tip: Follow a detailed cleaning protocol and double-check the setup before starting.
Performing the Study in Static Conditions
In static conditions, the smoke study aims to confirm that the airflow within the Class A/ISO 5 zones is unidirectional and cascades appropriately to areas with lower cleanliness requirements. The smoke should be introduced in a manner that allows it to merge seamlessly with the airflow, demonstrating a clear path that sweeps down and away from exposed products and critical surfaces. It is crucial to ensure that the smoke density does not obscure the view, allowing for clear visual confirmation of unidirectional airflow patterns.
Common Pitfall: Introducing too much or too little smoke.
Troubleshooting Tip: Use a controlled smoke generator and adjust the smoke density to ensure clear visibility without obscuring views.
Performing the Study in Dynamic Conditions
During dynamic conditions, the study focuses on verifying that various interventions by personnel or equipment do not disrupt the first air, ensuring that sterile surfaces and products remain uncontaminated. The smoke source should be positioned to follow the operator’s movements closely, providing continuous visualization of the impact on airflow.
All known interventions for each drug product-filling operation should be accurately simulated, and ergonomic considerations for different operators should be included to ensure a comprehensive assessment. The video recording of the study should be managed by a skilled director to minimize the need for reshoots and to ensure clear, accurate documentation of the airflow dynamics.
Common Pitfall: Not accurately simulating operational conditions.
Troubleshooting Tip: Ensure all typical activities and interventions are included in the simulation, and adjust the smoke introduction to follow personnel movements closely.
Analyzing and Documenting Results
A critical phase of the smoke study involves reviewing and recording the findings to ensure the cleanroom’s performance meets the required standards.
Interpreting Smoke Patterns
During smoke studies, the interpretation of smoke patterns is crucial for validating the unidirectional airflow and ensuring no contamination risks are present. Observations should include whether the smoke demonstrates laminarity over the product path and if there is a sweeping action away from the product path.
It is essential to ensure that the smoke flows continuously during the activity, and any turbulence observed in the smoke flow should not be ignored, as this could indicate airflow issues. Furthermore, the smoke should merge seamlessly with the airflow, showing no signs of disruption even when interventions are performed according to standard operating procedures.
Common Pitfall: Misinterpreting smoke behavior.
Troubleshooting Tip: Use multiple observers and video recordings to verify observations and ensure accurate interpretation.
Common Issues and Troubleshooting
In the analysis process, several common issues may arise, such as poor visibility due to reflection, poor lighting, dense smoke, or inappropriate camera angles. To address these issues, it is recommended to adjust the lighting, explore different camera angles, or modify the smoke density to ensure clear visibility of interventions.
If the smoke flow is interrupted during the activity, it is crucial to identify the cause and correct it to maintain continuous smoke flow. Additionally, any deviations from the expected smoke behavior, such as unexpected eddy currents or dead spaces, should prompt a thorough investigation to identify and mitigate the underlying causes.
Common Pitfall: Poor documentation of issues.
Troubleshooting Tip: Adjust lighting, camera angles, and smoke density as needed. Document all issues and corrective actions taken.
RELATED: CAPA Plan in GMP
Reporting Findings
The results of the smoke study should be meticulously documented, providing an unambiguous video record of the airflow patterns during both static and dynamic conditions. The final report should include detailed descriptions of each test condition, the equipment used, and the outcomes observed.
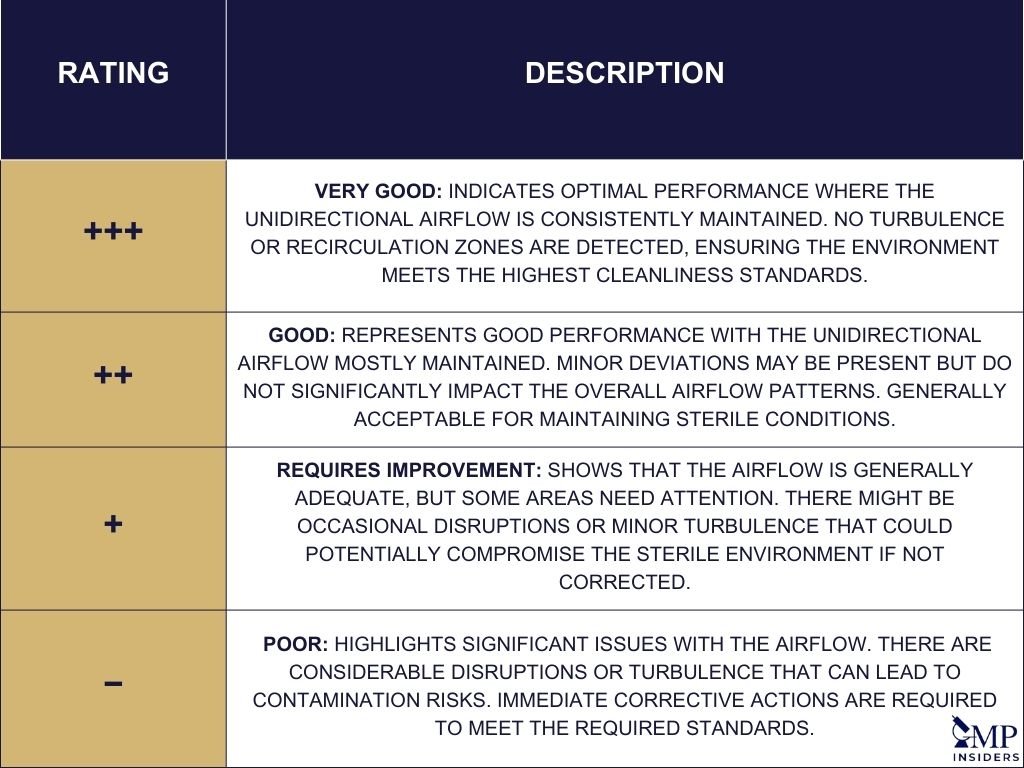
It is important to record all relevant data such as the date, time, personnel present, and room location at the time the smoke studies are conducted. The results should be reported in a binary fashion (Yes or No) or rated (Very Good “+++”, Good “++”, Requires Improvement “+”, Poor “-“) based on predefined acceptance criteria. This comprehensive documentation ensures that the airflow visualization studies meet all regulatory and quality standards, providing evidence that the cleanroom or barrier system operates as intended.
Common Pitfall: Incomplete or unclear documentation.
Troubleshooting Tip: Use a standardized reporting format, include all relevant data, and ensure clarity in describing findings.
FAQ
How Do You Prepare a Cleanroom Environment for a Static Airflow Visualization Test?
To prepare a clean room for a static airflow visualization test, the environment must be thoroughly cleaned to remove any particles or contaminants. All operations should be shut down, ensuring no personnel are present, and the HVAC systems should be allowed to stabilize. Entry points such as doors and windows must be sealed, and smoke generators should be set up in critical areas for the test.
Why Is It Important to Conduct Airflow Visualization Tests at Both Rest and Operational States?
Conducting airflow visualization tests at both rest and operational states is important because it ensures that the cleanroom meets airflow standards under different conditions. At-rest tests provide a baseline understanding of airflow patterns without personnel or operational activities, while operational tests validate that the cleanroom maintains its cleanliness standards during actual use, accounting for the presence of personnel and equipment.
How Do You Ensure That the Smoke Used in Visualization Tests Does Not Contaminate the Cleanroom Environment?
To ensure that the smoke used in visualization tests does not contaminate the cleanroom environment, non-toxic and non-contaminating smoke agents specifically designed for cleanroom use should be employed. These agents should be HEPA-filtered before introduction to remove any particles, and the cleanroom should be thoroughly cleaned after the test to eliminate any residual smoke particles.
What Criteria Are Used to Evaluate the Results of a Smoke Test in a Cleanroom?
The criteria used to evaluate the results of a smoke test in a cleanroom include verifying unidirectional airflow, ensuring there is no turbulence or stagnant air, and confirming that the airflow covers all critical areas uniformly. The consistency of the airflow pattern should be observed, and pressure differentials and HEPA filter integrity must be maintained throughout the test.
How Often Should Airflow Visualization Tests Be Performed to Maintain Compliance With Regulatory Standards?
Airflow visualization tests should be performed during the initial qualification of the cleanroom and its HVAC systems, periodically as part of routine requalification (typically every six months to a year), after any significant changes to the cleanroom, and following any contamination events or unexplained deviations in environmental monitoring data.
RELATED: Qualification vs Validation: Key Differences and Importance in the Pharmaceutical Industry
What Are the Key Indicators of Proper Unidirectional Airflow in a Cleanroom During a Smoke Test?
Key indicators of proper unidirectional airflow in a cleanroom during a smoke test include consistent flow in a single direction, even distribution of airflow across the entire cleanroom, and the absence of turbulence or stagnant zones. Additionally, the smoke should be quickly and efficiently cleared from the area, demonstrating effective contaminant removal.
How Do You Identify and Address Areas of Turbulence or Stagnant Air Detected During a Smoke Test?
Areas of turbulence or stagnant air can be identified by carefully observing the movement of smoke during the test and mapping any irregularities. To address these issues, potential causes such as improper equipment placement or HVAC system inadequacies should be investigated. Corrective actions may include repositioning equipment, adjusting airflow rates, or redesigning ductwork, followed by retesting to ensure the issues have been resolved.
Why Is It Essential to Perform Individual HEPA Filter Tests Using Smoke Visualization?
It is essential to perform individual HEPA filter tests using smoke visualization to verify that each filter is intact and free from leaks, ensuring proper air quality. These tests confirm that the airflow through each HEPA filter is uniform and adequate, and help detect any performance issues early, allowing for timely maintenance or replacement.
Conclusion
Airflow Visualization Smoke Studies (AVS) are vital for ensuring the sterility and quality of air in critical environments such as cleanrooms and aseptic processing areas. These studies help visualize and confirm proper airflow patterns, identify potential issues, and ensure compliance with cGMP (Current Good Manufacturing Practice) regulatory standards.
By understanding the various types of airflow visualization tests, including static and dynamic conditions, and by following a structured approach to planning and executing these tests, facilities can maintain high standards of air quality and operational efficiency.















