Cleaning validation is a crucial process in the pharmaceutical industry that ensures manufacturing equipment is effectively cleaned to prevent product contamination. Adhering to Current Good Manufacturing Practice (cGMP) and Quality Systems Regulations (QSR), cleaning validation as a critical component of quality assurance plays a vital role in ensuring that the manufacturing process remains not only efficient but also compliant with health and safety standards.
This article aims to provide a thorough understanding of cleaning validation and its role in the pharmaceutical industry, highlighting its critical steps and considerations in this vital process and the regulatory guidelines that govern the entire process.
What is Cleaning Validation?
Cleaning validation involves establishing evidence that cleaning processes effectively remove product residues and cleaning agents from equipment surfaces. It’s vital to prevent contamination and cross-contamination, ensuring product purity and effectiveness and patient safety.
The scope of cleaning validation extends to all equipment, facilities, and tools involved in the manufacturing process. It includes various steps, such as residue identification, detection and quantitation method selection, sampling method selection, setting residue acceptance criteria, method validation, and writing procedures for operators.
Common Pharmaceutical Terms in Cleaning Validation
Cleaning validation in the pharmaceutical industry involves various jargon and abbreviations that are important for manufacturing personnel to be familiar with. Here are twelve common terms related to cleaning validation and their definitions:
- CGMP – Current Good Manufacturing Practice: A set of regulations and guidelines established by regulatory agencies to ensure the quality and safety of pharmaceutical products.
- API – Active Pharmaceutical Ingredient: The active substance in a pharmaceutical product that provides the intended therapeutic effect.
- ADE – Acceptable Daily Exposure: The amount of a substance that can be safely consumed on a daily basis without adverse effects.
- PDE – Permitted Daily Exposure: The maximum allowable amount of a substance that can be consumed daily without adverse effects.
- MACO – Maximum Allowable Carry Over: The maximum amount of residue from one product that is allowed to carry over to another product during manufacturing processes.
- NOEL – No Observed Effect Level: The highest dose of a substance that does not result in any observed adverse effects in test animals.
- LOEL – Lowest Observed Effect Level: The lowest dose of a substance that results in observed adverse effects in test animals.
- NOAEL – No Observed Adverse Effect Level: The highest dose of a substance that does not result in any observed adverse effects in test animals.
- LOAEL – Lowest Observed Adverse Effect Level: The lowest dose of a substance that results in observed adverse effects in test animals.
- LOQ – Quantitation Limit: The lowest concentration of a substance that can be reliably measured and quantified.
- LOD – Detection Limit: The lowest concentration of a substance that can be reliably detected but not necessarily quantified.
- LD – Lethal Dose: The dose of a substance that is lethal or fatal to a specific percentage of a test population.
These terms are commonly used in the context of cleaning validation and ensure clear communication among various stakeholders involved in the process.
Regulatory Guidelines for Cleaning Validation
Regulatory bodies such as the FDA (Food and Drug Administration), EMA (European Medicines Agency), and WHO (World Health Organization) play a pivotal role in setting guidelines for cleaning validation. These guidelines ensure consistency and safety across the industry. Adhering to these regulations is not just about compliance; it’s about ensuring the highest quality of pharmaceutical products.
FDA
The FDA’s regulations, particularly 21 CFR Part 211, set the standard for cleaning validation in the U.S. CFR 211.67 specifically addresses the cleaning of equipment, emphasizing the need for written procedures that prevent contamination and cross-contamination. This section mandates proper maintenance, cleaning, and sanitizing of equipment at appropriate intervals.
Inspection References and Q&A on Current Good Manufacturing Practices
The FDA also provides a “Guide to Inspections Validation of Cleaning Processes” and a series of Questions & Answers on Current Good Manufacturing Practices—Equipment. These resources offer practical insights into FDA expectations during inspections and clarify aspects of equipment-related GMPs.
EMA
The EMA requires the establishment of Health-Based Exposure Limits (HBELs) for drug products and emphasizes a risk-based approach to cleaning validation. Compliance with these guidelines is crucial to ensure the safety and quality of pharmaceutical products.
Annex 15: Qualification and Validation
EMA’s guidelines for cleaning validation are encapsulated in Annex 15, sections 10.1 to 10.15. They detail the requirements for validating cleaning procedures, ensuring they are reliably removing residues to predetermined acceptable levels.
Related article: Qualification vs Validation
EMA Cleaning Validation Guideline on Setting HBELs
The EMA also provides specific guidelines on setting Health Based Exposure Limits (HBELs). These limits are crucial for assessing the risk of cross-contamination and ensuring the safety of pharmaceutical products.
WHO
WHO’s Annex 3 focuses on validation and qualification processes, including cleaning validation. It serves as an international standard, especially for countries developing their regulatory frameworks.
ISPE
ISPE’s Baseline Guide Vol 7, titled “Risk-Based Manufacture of Pharmaceutical Products 2nd Edition” and their Cleaning Validation Lifecycle guideline offer comprehensive insights into risk-based approaches and the lifecycle concept in cleaning validation.
PIC/S
PIC/S guideline Cross-Contamination in Shared Facilities – PI-043-1 addresses the risks associated with cross-contamination in shared facilities. It offers a framework for managing these risks, especially in multiproduct manufacturing environments.
PIC/S Guideline on Setting HBEL for Shared Facilities
This guideline complements the EMA’s approach, providing additional insights on setting HBELs for risk identification in shared facilities. It’s a crucial resource for managing cross-contamination risks in multi-product environments.
Parenteral Drug Association (PDA)
PDA Technical Report 29, “Points to Consider for Cleaning Validation” and Report 49, focusing on biotechnology cleaning validation, provide industry-specific insights and considerations. These reports are particularly valuable for manufacturers of parenteral drugs.
American Society For Testing and Materials (ASTM)
ASTM’s E3106 – 18e1 is a standard guide for science-based and risk-based cleaning process development and validation. Additionally, their guide for the derivation of HBELs complements the guidelines set by EMA and PIC/S, focusing on the establishment of safe exposure limits.
Steps Involved in Cleaning Validation Process

The cleaning validation process consists of several sequential steps to ensure that equipment and processes are thoroughly cleaned and free from any residual contaminants. These steps can be summarized as follows:
Defining the Scope and Objectives of a Cleaning Validation Program
The initial step in designing a cleaning validation program involves defining its scope and objectives. This foundational phase sets the direction for the entire validation process, ensuring that it is both comprehensive and focused on critical areas.
Selection of Worst Case Scenario in Cleaning Validation
In cleaning validation for the pharmaceutical industry, the concept of a “worst-case scenario” plays a critical role. Selecting and testing under worst-case conditions ensures the cleaning process is effective under all possible circumstances. This approach is pivotal for guaranteeing patient safety and product quality.
RELATED: Worst Case Selection in Cleaning Validation
Risk Assessment
Before initiating the cleaning validation process, manufacturers should conduct a risk assessment to identify potential sources of contamination and determine the level of risk associated with each source. This assessment helps prioritize cleaning efforts and focus on critical areas that pose the highest risk to product quality and patient safety.
Cleaning Procedure Development
Once the risks are identified, manufacturers need to develop cleaning procedures that are specific to each piece of equipment or process. These procedures should outline the appropriate cleaning agents, cleaning methods, and cleaning frequencies. They should also consider factors such as material compatibility, cleaning validation limits, and regulatory requirements.
Sampling Plan and Analytical Methods
Manufacturers should establish a sampling plan to determine the locations and frequency of sampling during the cleaning process. The plan should consider both visual inspection and analytical testing to ensure that all residues and contaminants are effectively removed. Analytical methods, such as chromatography or spectroscopy, are commonly used to detect and quantify residues.
Validation Protocol Development
Manufacturers should develop a validation protocol that outlines the specific tests and acceptance criteria for each cleaning procedure. The protocol should include details such as sample size, sampling locations, analytical methods, and acceptance limits. It should also specify the number of validation runs required to establish the effectiveness of the cleaning process. Regular training of operators on the protocol is critical to ensure consistent and accurate execution of cleaning validation procedures.
Execution of Cleaning Validation
Once the cleaning procedures, sampling plan, and validation protocol are established, manufacturers can execute the cleaning validation process. This involves performing the cleaning procedure as per the developed protocol, collecting samples at designated locations, and analyzing the samples for residual contaminants. The cleaning process is repeated for the required number of validation runs to ensure consistency and reproducibility.
Data Analysis and Reporting
After completing the cleaning validation runs, manufacturers need to analyze the data obtained from the analytical testing. This analysis involves comparing the results against the acceptance criteria specified in the validation protocol. If the results meet the acceptance criteria, the cleaning process is considered validated. A comprehensive report documenting the cleaning validation process, including all procedures, results, and conclusions, should be prepared and maintained for regulatory purposes.
The cleaning validation process is iterative, and any deviations or failures detected during the validation runs should be investigated and addressed before the cleaning process is considered validated. This ensures that the cleaning procedures are robust, effective, and capable of consistently removing residues and contaminants.
Sampling Methods for Cleaning Validation
Sampling methods play a vital role in cleaning validation as they determine how residues are detected and measured. Common sampling methods include rinse water sampling, swab or wipe sampling, coupon sampling, placebo sampling, and direct analysis.
Swab sampling is a straightforward sampling method where a sterile swab is used to wipe a specific section of the equipment’s surface. The swab is then subjected to extraction and analysis to detect any residual substances.
Rinse sampling is a method of sampling that’s performed indirectly. It entails the collection and subsequent analysis of the rinse water or solvent used in the post-cleaning wash of the equipment.
Coupon sampling is a combined method of sampling that utilizes a small material piece, known as a coupon, which replicates the surface of the equipment. This coupon is exposed to both the product and the cleaning agent. After exposure, the coupon is removed and examined for any residual substances.
Placebo sampling uses placebo products to analyze residues from previous batches, and direct analysis involves taking residual readings directly from the surface of equipment using specialized instruments.
Types of Cleaning Methods in Cleaning Validation
Various cleaning methods are employed in cleaning validation within the pharmaceutical industry. These methods are designed to remove residues and contaminants from equipment surfaces effectively. Some of the most commonly used cleaning methods include:
- Clean-in-place (CIP) Method: This method involves using fixed or rotating spray devices with a wash tank, recirculation pump, and associated piping to clean equipment in place.
- Clean-out-of-place (COP) Method: COP is often used for automated parts washing through cabinet or tunnel washers with cleaning, rinsing, and drying cycles.
- SIP (Sterilize-In-Place): Similar to CIP but involves sterilizing agents. It’s essential for equipment used in sterile processing.
- Immersion Method: The immersion method can be either agitated, where a cleaning agent in a process vessel is mechanically stimulated, or static, where the process vessel is soaked with the cleaning agent.
- Ultrasonic Washing: Ultrasonic washing is particularly effective for intricate parts, such as filling needles. It involves using a tank equipped with ultrasonic transducers to induce cavitation, which helps remove residues.
- High-pressure Spraying: High-pressure spraying is employed to dislodge residues on surfaces using high-pressure, continuous, and directed water or cleaning solution.
- Manual Cleaning: Manual cleaning is often considered the most challenging method to validate. It includes techniques such as wiping, sink brushing, and equipment brushing.
These cleaning methods play a crucial role in ensuring that equipment surfaces are thoroughly cleaned and free from contaminants, thus minimizing the risk of product contamination.
Criteria for Selecting a Cleaning Method
The choice of a cleaning method in the pharmaceutical industry is a critical decision. It depends on various factors:
- Type of Residue: The nature of the residue (e.g., oil-based, aqueous, solid) dictates the cleaning agent and method.
- Equipment Compatibility: The selected method must not damage the equipment. Factors like material of construction and design limitations are considered.
- Effectiveness: The method should effectively remove residues to the required levels without leaving its own residues.
- Efficiency: Time and resource efficiency are crucial to maintain productivity.
- Safety: Methods should not pose risks to operators or the environment.
- Regulatory Compliance: The method must adhere to the guidelines set by regulatory bodies.
Acceptance Criteria for Cleaning Validation
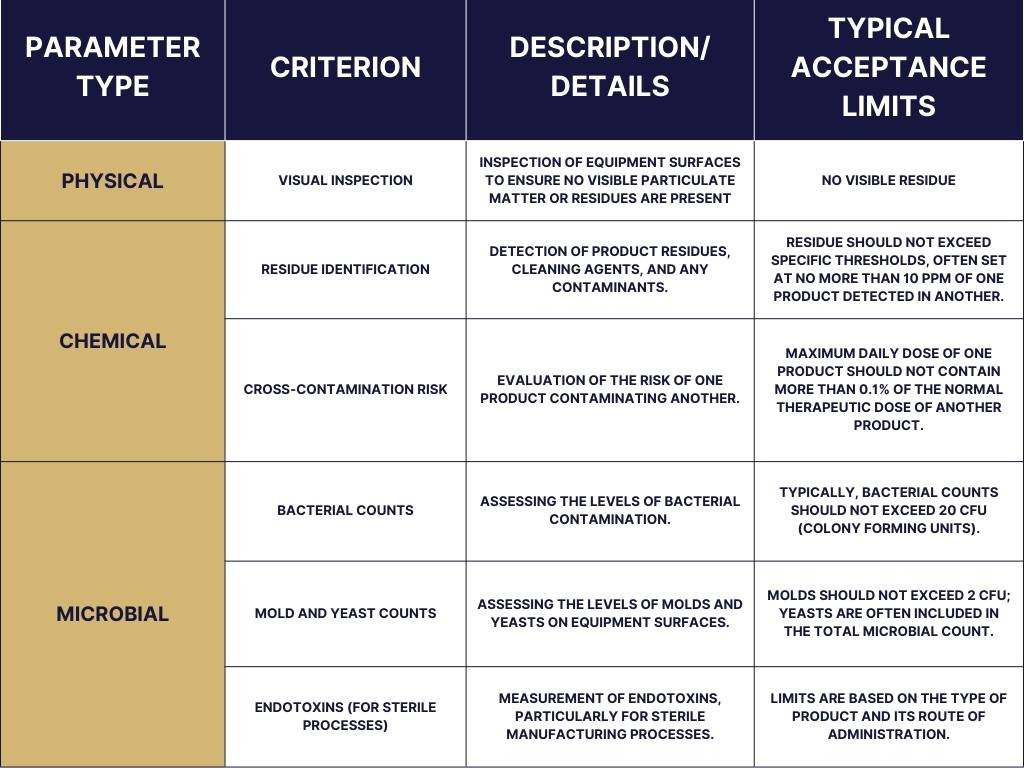
Setting accurate acceptance criteria is a critical aspect of cleaning validation. Acceptance criteria determine whether the cleaning process is effective and meets the required standards. Acceptance criteria in cleaning validation can be categorized into three testing parameters: physical, chemical, and microbial criteria.
- Physical Criterion: The visual inspection of equipment surfaces should reveal no visible particulate matter or residues.
- Chemical Criterion: The presence of one product in another product should not exceed 10 parts per million (ppm), and the maximum daily dose of one product should not contain more than 0.1% of the normal therapeutic dose of another product.
- Microbial Criterion: Bacterial counts should not exceed 20 colony-forming units (CFU), molds should not exceed 2 CFU, and contaminants should not exceed 25 CFU/25cm2 in a sample.
By establishing these acceptance criteria, pharmaceutical companies can ensure that the cleaning processes effectively remove residues and contaminants, meeting the required standards for product safety and quality.
Best Cleaning Validation Practices
To ensure the effectiveness of cleaning validation, it is essential to follow best practices. Some key best practices include:
- Adherence to regulatory guidelines: Stay updated with the latest regulatory requirements and guidelines to ensure compliance.
- Establishing a Robust Cleaning Program: The foundation of successful cleaning validation is a robust cleaning program that includes well-documented cleaning procedures, appropriate cleaning agents, and validated cleaning methods. Manufacturers should invest time and resources in developing comprehensive cleaning procedures that address all critical areas and potential sources of contamination.
- Risk-Based Approach: Employing a risk-based approach helps prioritize cleaning efforts and resources. Manufacturers should conduct a thorough risk assessment to identify critical areas and focus on them during the cleaning validation process. This approach ensures that cleaning efforts are targeted where they are most needed, minimizing the risk of contamination.
- Validation Master Plan: Developing a validation master plan that outlines the overall validation strategy, including cleaning validation, is crucial for maintaining consistency and compliance. The plan should define the scope, objectives, and responsibilities for each validation activity and provide a roadmap for the entire validation process.
- Regular Monitoring of Cleaning Effectiveness: Cleaning processes should be regularly monitored to ensure their ongoing effectiveness. This can be achieved through routine visual inspections, as well as periodic swab testing and analytical analysis. Monitoring provides valuable data on the performance of cleaning procedures and helps identify any deviations or failures that need to be addressed.
- Effective Training and Documentation: Proper training of personnel involved in cleaning validation is essential to ensure consistent execution of cleaning procedures. Training should cover the importance of cleaning validation, proper cleaning techniques, and the use of appropriate cleaning agents. Additionally, comprehensive documentation of all cleaning validation activities, including protocols, results, and conclusions, is necessary to demonstrate compliance with regulatory requirements.
- Collaboration with cleaning product suppliers: Work closely with cleaning product suppliers to obtain expert guidance, support, and validated cleaning products.
FAQ
Is Cleaning Validation Required for Dedicated Equipment?
Yes, cleaning validation is required for all equipment and manufacturing systems used in the production, processing, packing, or holding of drug products, including dedicated equipment. The FDA pays particular attention to dedicated equipment as they can be more difficult to clean, and the risk of contamination is higher.
How Is the Worst Case Selected in Cleaning Validation?
The selection of the worst case for cleaning validation is typically based on factors such as drug solubility, the difficulty of equipment cleaning, and the occupancy of products in the production line. Various criteria, such as drug solubility in water, are used to determine the worst-case scenario and guide the cleaning validation process.
What Is the 10 ppm Criteria for Cleaning Validation?
The 10 ppm criteria for cleaning validation is a widely accepted standard in the pharmaceutical industry, which stipulates that no more than 10 parts per million of any product residue should be present on manufacturing equipment after cleaning. This threshold ensures that residual contamination is kept to a minimal, safe level, thereby protecting the purity and quality of subsequent pharmaceutical products.
What Are Common Challenges in Cleaning Validation for Sterile Manufacturing?
Cleaning validation challenges in sterile manufacturing include validating cleaning procedures for complex equipment, ensuring the removal of all types of contaminants, and maintaining consistency in cleaning practices.
Conclusion
Cleaning validation is a critical process in the pharmaceutical industry to ensure product quality, safety, and compliance with regulatory requirements. By following established guidelines, selecting appropriate sampling methods, setting realistic acceptance criteria, and collaborating with cleaning product suppliers, pharmaceutical manufacturers can effectively validate their cleaning processes.
Continued advancements in cleaning validation processes, automation, and analytical techniques will further enhance the efficiency and effectiveness of cleaning validation in the future. By staying informed and adopting best practices, pharmaceutical manufacturers can maintain high standards of cleanliness, protect patient safety, and ensure the quality of their products in an ever-evolving industry.














