Cold chain management is vital in the pharmaceutical industry to ensure the safety and efficacy of temperature-sensitive products such as vaccines and biologics. This involves maintaining specific temperature ranges during storage, transportation, and handling to prevent product degradation.
Effective cold chain management encompasses temperature-controlled storage facilities, specialized transport vehicles, and continuous temperature monitoring. Adhering to regulatory standards set by the FDA, EMA, and WHO is crucial to ensure compliance and maintain product quality.
This article will explore the key components, regulatory standards, technologies, best practices, and challenges in cold chain management within the pharmaceutical industry.
What is Cold Chain Management?
Cold chain management within Good Manufacturing Practice (GMP) and Good Distribution Practice (GDP) is defined as managing the storage and transportation of temperature-sensitive products, such as pharmaceuticals, to maintain their integrity and quality throughout the supply chain.
This system is crucial for preventing spoilage, contamination, and degradation of products. Ensuring that these products are safe from these risks is vital for maintaining their efficacy and safety, which is essential for patient health and regulatory compliance.
RELATED: Good Distribution Practice (GDP)
Critical Elements
Some of the critical elements for cold chain management include:
- Storage Solutions: Effective cold chain management starts with the proper storage solutions, which may include specialized rooms, chillers, refrigerators, or insulated containers. These are crucial for maintaining the required temperature-controlled environments for sensitive pharmaceutical products.
- Thermal Packaging: The design of thermal packaging plays a significant role in maintaining the correct temperature during transportation. It is essential that the packaging used is specifically suited to the product’s requirements to prevent degradation.
- Temperature Monitoring and Control: Utilizing advanced technologies such as data loggers, temperature indicators, and live sensors enables real-time monitoring and control of temperature conditions. This allows for immediate detection and response to any deviations or issues in the cold chain process.
- Transportation: The transportation of products in a cold chain involves the use of specialized vehicles and containers that are equipped to maintain the necessary temperature conditions. Ensuring that these conditions are consistently met, especially during long-distance or international transport, is crucial for the integrity of the products.
By integrating these critical elements, organizations can effectively manage their cold chain processes within GMP environments, thereby safeguarding the quality and safety of their temperature-sensitive products.
Key Components of Pharmaceutical Cold Chain
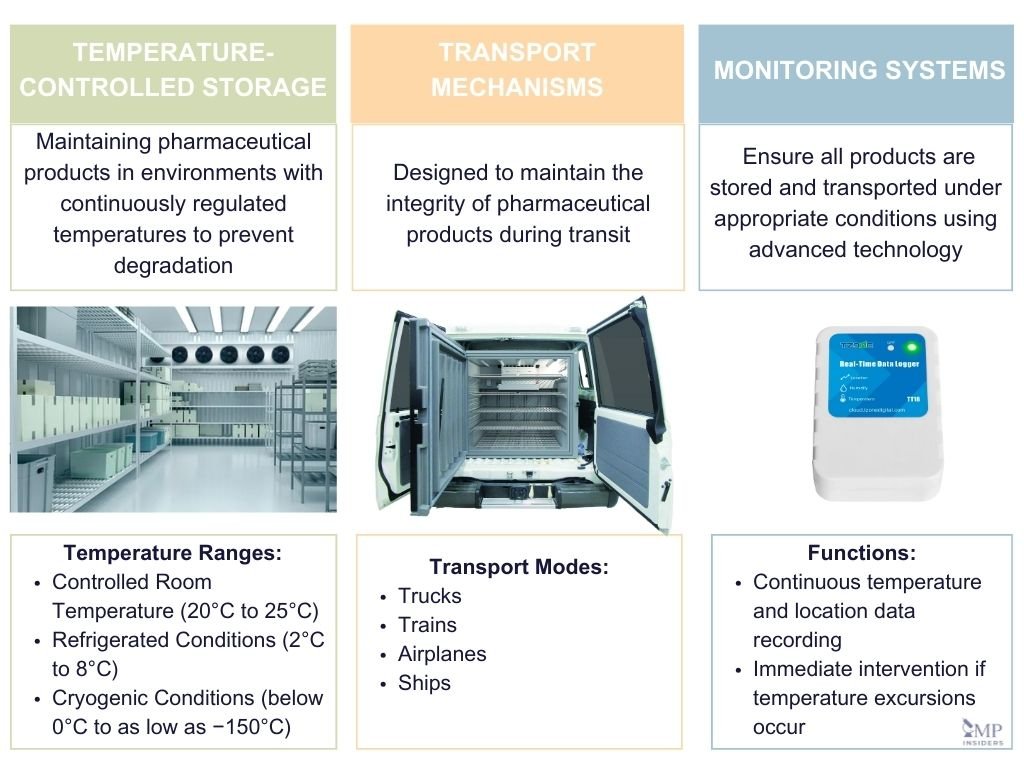
Some of the key components of the pharmaceutical cold chain include:
Temperature-Controlled Storage
Temperature-controlled storage is a critical component of pharmaceutical cold chain management. It involves maintaining pharmaceutical products in environments with continuously regulated temperatures to prevent product degradation. This includes the use of refrigerated warehouses, cold rooms, and specialized refrigeration units.
These facilities must adhere to specific temperature ranges, such as controlled room temperature (20°C to 25°C), refrigerated conditions (2°C to 8°C), and cryogenic conditions (below 0°C to as low as −150°C) depending on the product requirements. Additionally, the storage areas are equipped with monitoring systems that ensure compliance with regulatory standards and maintain the efficacy and safety of the pharmaceuticals.
Transport Mechanisms
The transport mechanisms in the pharmaceutical cold chain are designed to maintain the integrity of pharmaceutical products during transit. This includes the use of refrigerated trucks, temperature-controlled shipping containers, and thermal packaging solutions.
Different transport modes such as trucks, trains, airplanes, and ships are utilized based on the distance and the specific requirements of the pharmaceutical products. Each mode is equipped with temperature control systems to handle various temperature ranges, from ambient and refrigerated to cryogenic conditions, ensuring that products like vaccines and biologics are transported under optimal conditions.
See Also: Transport Validation in GDP
Monitoring Systems
Monitoring systems play a pivotal role in the pharmaceutical cold chain by ensuring that all products are stored and transported under appropriate conditions. These systems utilize advanced technology to record and monitor temperature data continuously throughout the supply chain.
Common monitoring solutions include USB dataloggers and real-time cellular monitoring devices, which provide data on temperature and location, allowing for immediate intervention if temperature excursions occur. These systems are crucial for maintaining regulatory compliance and for providing evidence that pharmaceutical products have been handled correctly throughout their journey.
Additionally, the integration of GPS trackers and real-time temperature sensors helps in managing the logistics of temperature-sensitive products more effectively, ensuring their quality and safety upon delivery.
Regulatory Standards and Guidelines
Maintaining regulatory compliance in the cold chain is complex and critical. Pharmaceutical companies are required to adhere to stringent regulations outlined by bodies such as the European and United States Pharmacopeia, which hold manufacturers accountable for the quality of their products until they reach the patient.
Compliance involves not only maintaining temperature within specified limits but also ensuring that all handling and transportation processes meet regulatory standards.
The compliance challenges are compounded by the need for detailed documentation and the ability to respond to temperature excursions with appropriate corrective actions. Manufacturers must have procedures to document, investigate, and handle temperature excursions, following Good Distribution Practices (GDP) and cGMP regulations.
FDA
The FDA provides comprehensive guidelines under the Good Distribution Practice (GDP) for maintaining the quality and safety of pharmaceutical products:
- 21 CFR Part 211: Addresses current Good Manufacturing Practices (cGMP) for finished pharmaceuticals, including requirements for temperature control and monitoring during storage and transportation.
- 21 CFR 205.50: Sets the minimum requirements for the storage and handling of prescription drugs and maintaining distribution records.
- 21 CFR 203.32: Outlines the requirements for drug sample storage and handling to maintain stability, integrity, and effectiveness as per the manufacturer’s specifications.
- FDA Guidance for Industry: Process Validation: General Principles and Practices: Includes recommendations for the validation of equipment used in the cold chain.
EMA
The EMA outlines standards for the distribution of medicinal products within the EU under the following guidelines:
- EU Guidelines for Good Distribution Practice of Medicinal Products for Human Use (2013/C 343/01): Specifies requirements for maintaining temperature control during storage and transportation.
- European Commission’s Guideline 2015/C 95/01: Ensures that medicinal products are distributed to the end user without any compromise in quality.
- Regulation (EC) No 178/2002: Provides a framework for ensuring food safety, which can be relevant for certain temperature-sensitive medicinal products.
- EU Annex 11: Provides a framework for managing electronic records related to medicinal product distribution.
WHO
The WHO provides international guidelines for the distribution of vaccines and other temperature-sensitive pharmaceuticals:
- WHO Technical Report Series, No. 961, Annex 9: Model guidance for the storage and transport of time- and temperature-sensitive pharmaceutical products (2011): Details the requirements for maintaining proper storage and transportation conditions for vaccines.
- WHO Guidelines on the International Packaging and Shipping of Vaccines (2005): Includes recommendations for temperature control and packaging during vaccine transportation.
US Pharmacopeia (USP)
The USP sets forth best practices for storing and handling temperature-sensitive medications:
- USP General Chapter <1079>: Good Storage and Distribution Practices for Drug Products: Provides guidelines for the proper storage and distribution of pharmaceuticals, including temperature control.
- USP General Chapter <659>: Packaging and Storage Requirements: Specifies requirements for packaging and storage conditions to protect products from temperature fluctuations.
- USP General Chapter <1118>: Monitoring Devices—Time, Temperature, and Humidity: Provides guidance on the selection, qualification, and use of monitoring devices for time, temperature, and humidity.
ASTM
The ASTM develops standards for the design and performance of temperature-controlled packaging and transportation systems:
- ASTM D3103-20: Standard Test Method for Thermal Insulation Performance of Distribution Packaging: Outlines testing protocols to ensure packaging maintains required temperatures.
- ASTM D4169-22: Standard Practice for Performance Testing of Shipping Containers and Systems: Includes procedures for testing the performance of packaging systems under various environmental conditions, including temperature control.
ISPE
The ISPE provides best practices and guidelines to ensure the integrity of the cold chain in the pharmaceutical industry:
- ISPE Good Practice Guide: Cold Chain Management: Offers comprehensive guidelines for the design, implementation, and management of cold chain systems.
- ISPE Baseline Guide: Commissioning and Qualification: Includes strategies for commissioning and qualifying cold chain equipment and systems to ensure they meet regulatory and performance requirements.
Technologies and Innovations Used for Cold Chain Management
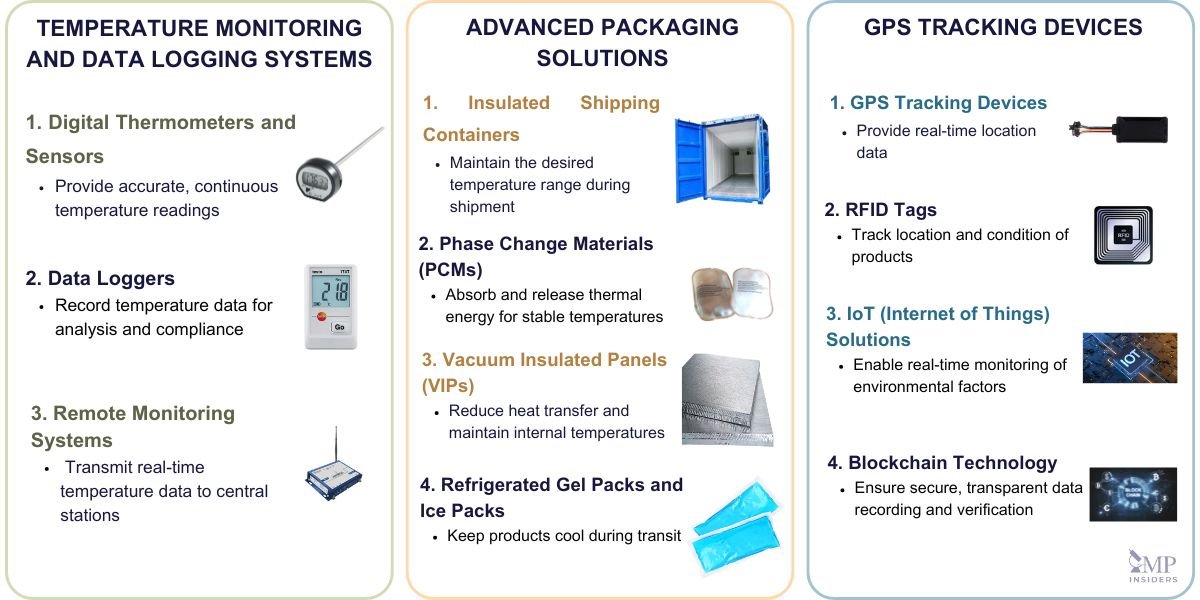
Advancements in technology ensure precise temperature control, real-time tracking, and overall efficiency throughout the supply chain. Some of the key technologies and innovations include:
Temperature Monitoring and Data Logging Systems
Temperature monitoring and data logging systems are essential for maintaining the integrity of temperature-sensitive pharmaceuticals. These systems continuously record temperature data throughout storage and transportation, providing real-time alerts and comprehensive reports to ensure compliance with regulatory standards.
- Digital Thermometers and Sensors: Advanced digital thermometers and sensors provide accurate and continuous temperature readings, ensuring immediate detection of any deviations.
- Data Loggers: Data loggers record temperature data at regular intervals, storing the information for later analysis and regulatory compliance. Examples include single-use and multi-use loggers with various features like wireless data transmission.
- Remote Monitoring Systems: These systems use wireless technology to transmit real-time temperature data to central monitoring stations, allowing for instant alerts and remote management.
Advanced Packaging Solutions
Innovative packaging solutions are crucial for maintaining temperature stability during the transportation of pharmaceuticals. These solutions are designed to protect products from external temperature fluctuations and ensure consistent conditions throughout the journey.
- Insulated Shipping Containers: These containers are equipped with advanced insulation materials to maintain the desired temperature range. They come in various sizes and specifications to cater to different shipment volumes and requirements.
- Phase Change Materials (PCMs): PCMs absorb and release thermal energy during phase transitions (e.g., from solid to liquid), providing a stable temperature environment within the packaging.
- Vacuum Insulated Panels (VIPs): VIPs offer superior insulation properties, significantly reducing heat transfer and maintaining internal temperatures for extended periods.
- Refrigerated Gel Packs and Ice Packs: Used to keep products cool during transit, these packs are designed to maintain specific temperatures and are often used in conjunction with other packaging solutions.
Real-Time Tracking Technologies
Real-time tracking technologies provide comprehensive visibility and control over the cold chain, ensuring that temperature-sensitive products are continuously monitored and managed effectively.
- GPS Tracking Devices: GPS-enabled devices provide real-time location data, allowing for precise tracking of shipments and ensuring they follow the planned route and schedule.
- RFID Tags: Radio-frequency identification (RFID) tags can be used to track the location and condition of pharmaceutical products. These tags store data that can be read remotely, providing detailed information on the product’s history and condition.
- IoT (Internet of Things) Solutions: IoT devices integrate various sensors and tracking technologies to provide a holistic view of the cold chain. They enable real-time monitoring of temperature, humidity, and other environmental factors, ensuring immediate response to any deviations.
- Blockchain Technology: Blockchain offers a secure and transparent method for recording and verifying transactions and data across the cold chain. It ensures data integrity and allows stakeholders to access real-time information on the status and history of products.
These systems not only help in maintaining product integrity but also optimize the management of the cold chain by reducing waste and improving response times.
Challenges in Cold Chain Management
Managing the cold chain for temperature-sensitive pharmaceuticals presents numerous challenges that can impact the integrity and efficacy of these products. Here are some of the key challenges:
Environmental Challenges
Environmental factors can significantly affect the cold chain, making it essential to implement robust measures to mitigate these challenges.
- Temperature Fluctuations: Variations in ambient temperature during transportation and storage can compromise product stability. Extreme weather conditions, such as heat waves or cold snaps, pose additional risks.
- Humidity and Moisture Control: High humidity levels can affect the packaging and potentially lead to product degradation. Maintaining appropriate humidity levels is crucial in certain climates and environments.
- Geographical Variations: Different regions have varying environmental conditions, which can complicate maintaining a consistent temperature throughout the supply chain.
Logistical Challenges
The logistical complexities of managing a global cold chain can pose significant challenges.
- Transportation Modalities: Coordinating multiple transportation modes, such as air, sea, and land, can lead to temperature excursions due to varying environmental conditions and handling practices.
- Customs and Regulatory Compliance: Navigating different countries’ customs regulations and ensuring compliance with varying regulatory standards can cause delays and disruptions.
- Infrastructure Limitations: Inadequate infrastructure, such as a lack of temperature-controlled storage facilities or reliable transportation options in certain regions, can hinder effective cold chain management.
- Coordination Among Stakeholders: Ensuring seamless coordination and communication among all stakeholders, including manufacturers, logistics providers, and distributors, is critical to maintaining the cold chain’s integrity.
- Emergency Situations: Unplanned events, such as natural disasters, political unrest, or pandemics, can disrupt transportation routes and affect the cold chain.
Temperature Excursions
Temperature excursions represent a significant challenge in GMP and GDP cold chain management. These occur when temperature-sensitive pharmaceutical products are exposed to temperatures outside the prescribed range for storage and transport, potentially compromising their efficacy and safety.
- WHO Definition: The World Health Organization defines a temperature excursion as an event in which a Time Temperature Sensitive Pharmaceutical Product (TTSPP) is exposed to temperatures outside the prescribed ranges.
- Causes: Factors contributing to temperature excursions include system failures, human error, inadequate air handling units (AHU) in manufacturing areas, mechanical failures, unexpected delays in transportation, improper handling at shipping yards, and the use of non-refrigerated vehicles.
- Consequences: Temperature excursions can lead to product loss, decreased efficacy, and potential adverse reactions in patients.
To manage these challenges, pharmaceutical companies must implement robust monitoring systems and maintain strict control over the environmental conditions throughout the product’s lifecycle. This includes the use of data loggers, sensors, and thermal packaging to ensure continuous temperature control and immediate response to any deviations.
Regulatory Compliance
Maintaining regulatory compliance in the cold chain is complex and critical. Pharmaceutical companies are required to adhere to stringent regulations outlined by regulatory bodies such as the European and United States Pharmacopeia, which hold manufacturers accountable for the quality of their products until they reach the patient.
- Compliance Requirements: Compliance involves maintaining temperature within specified limits and ensuring that all handling and transportation processes meet regulatory standards.
- Documentation and Corrective Actions: The compliance challenges are compounded by the need for detailed documentation and the ability to respond to temperature excursions with appropriate corrective actions. This includes developing a Corrective Action & Preventive Action (CAPA) system to address and mitigate any issues that arise.
- Transparency and Monitoring: The lack of end-to-end transparency in the supply chain and rapid environmental changes pose additional compliance risks. Pharmaceutical companies must invest in advanced technologies and systems that provide real-time tracking and monitoring to ensure that all aspects of the cold chain are visible and controlled.
- Regular Audits and Updates: Regular audits and updates to quality management systems are essential to adapt to evolving regulations and maintain compliance.
By addressing these challenges through strategic planning, technological investment, and rigorous compliance practices, pharmaceutical companies can ensure the integrity and efficacy of their products, ultimately safeguarding patient health.
Best Practices for Effective Cold Chain Management
Implementing best practices is essential for maintaining the integrity of temperature-sensitive pharmaceuticals throughout the cold chain. Here are key strategies and approaches:
Risk Assessment and Mitigation Strategies
Conducting thorough risk assessments and implementing mitigation strategies is crucial for preventing potential issues in the cold chain.
- Identify Potential Risks: Analyze each step of the cold chain to identify potential risks, such as temperature excursions, equipment failures, and transportation delays.
- Develop Mitigation Plans: Create detailed plans to address identified risks, including contingency measures and corrective actions.
- Regular Reviews: Periodically review and update risk assessments and mitigation plans to reflect changes in processes, technology, and regulations.
RELATED: Quality Risk Management (QRM)
Establishing Robust SOPs
Standard Operating Procedures (SOPs) are vital for ensuring consistency and compliance in cold chain management.
- Develop Comprehensive SOPs: Create SOPs covering all aspects of cold chain management, including storage, handling, transportation, monitoring, and documentation.
- Ensure Clarity and Accessibility: Ensure that SOPs are clear, concise, and easily accessible to all relevant personnel.
- Regular Updates: Regularly review and update SOPs to incorporate new technologies, regulatory changes, and process improvements.
Training Personnel
Well-trained personnel are essential for effective cold chain management.
- Comprehensive Training Programs: Develop training programs that cover the principles of cold chain management, regulatory requirements, and the use of relevant technologies.
- Regular Training Sessions: Conduct regular training sessions to keep personnel updated on best practices and any changes in procedures or regulations.
- Assessment and Certification: Implement assessment and certification processes to ensure that personnel understand and can effectively apply their training.
Continuous Monitoring and Quality Assurance
Continuous monitoring and quality assurance ensure that the cold chain remains intact and compliant with regulatory standards.
- Implement Monitoring Systems: Use advanced monitoring systems to continuously track temperature and other critical parameters throughout the cold chain.
- Data Analysis and Reporting: Regularly analyze monitoring data to identify trends, potential issues, and areas for improvement. Generate comprehensive reports for internal review and regulatory compliance.
- Quality Audits: Conduct regular quality audits to ensure that all aspects of the cold chain comply with SOPs and regulatory standards.
Partnering with Reliable Logistics Providers
Selecting reliable logistics providers is crucial for maintaining the integrity of the cold chain.
- Evaluate Providers: Carefully evaluate potential logistics providers based on their experience, reputation, and compliance with regulatory standards.
- Establish Clear Contracts: Develop clear contracts outlining the responsibilities and expectations of both parties, including temperature control, monitoring, and reporting requirements.
- Continuous Collaboration: Maintain ongoing communication and collaboration with logistics providers to address any issues promptly and ensure continuous improvement.
FAQ
How Do Phase Change Materials (PCMs) Work in Cold Chain Packaging?
PCMs absorb and release thermal energy during phase transitions, maintaining a stable temperature environment inside the packaging.
What Is the Difference Between Passive and Active Cold Chain Systems?
Passive systems use insulated containers and refrigerants without external power, while active systems use powered refrigeration units to maintain temperature.
What Is the Significance of Temperature Mapping in Cold Chain Management?
Temperature mapping identifies temperature variations within storage areas or transport vehicles, ensuring uniform temperature distribution and identifying hotspots.
How Do You Handle Cold Chain Management During Emergency Situations?
Emergency plans should include backup power sources, alternative transport routes, and contingency measures for maintaining temperature control during disruptions.
Conclusion
Cold chain management is a critical aspect of the pharmaceutical industry, essential for maintaining the safety, efficacy, and quality of temperature-sensitive products such as vaccines and biologics.
By maintaining strict temperature controls and leveraging advanced technologies, pharmaceutical companies can prevent product degradation and ensure regulatory compliance. Effective cold chain management not only safeguards product quality but also protects patient health, making it a critical aspect of modern pharmaceutical logistics.














