The integration of Corrective And Preventive Action (CAPA) systems within Good Manufacturing Practice (GMP) is fundamental for the pharmaceutical industry to maintain high standards of product quality and safety. As regulatory requirements become increasingly stringent, the role of CAPA in identifying, correcting, and preventing deviations from quality standards has never been more critical.
This article provides a comprehensive overview of CAPA’s significance in the GMP framework, outlining its key processes, implementation challenges, and effective strategies. Our goal is to offer actionable insights for industry professionals seeking to enhance their quality management practices and ensure regulatory compliance.
What is CAPA?
CAPA, or Corrective Action and Preventive Action, stands as a cornerstone in achieving cGMP compliance, particularly within the pharmaceutical industry. It encompasses a spectrum of activities designed to identify, investigate, and rectify product and quality issues while implementing steps to curb their recurrence, ensuring continuous improvement and conformity to GMP standards.
This importance is reflected through a systematic approach aimed at resolving issues that could negatively affect product quality, safety, or compliance. Leveraging methodologies like root cause analysis, CAPA aids in eliminating non-conformities or undesired events and prevents their occurrence through proactive risk management. The process not only mitigates regulatory risks by aligning with Quality Management Systems but also fosters a culture of continuous improvement, thereby streamlining compliance with GMP regulations and enhancing overall product quality.
Corrective Actions
Corrective actions are reactive measures taken to address problems after they have occurred, intending to prevent their recurrence. They are typically triggered by non-conformances, defects, or other identified issues affecting product quality, safety, or regulatory compliance.
Examples of Corrective Actions in the Pharmaceutical Industry
- Modification of Production Processes: If a batch failure occurs due to an inefficiency in the production process, a corrective action might involve modifying the process to enhance its reliability and consistency, such as upgrading to more precise mixing equipment.
- Revision of SOPs: After a safety incident in a manufacturing plant, an investigation reveals that existing Standard Operating Procedures (SOPs) did not adequately address certain safety risks. The corrective action involves revising the SOPs to include new safety measures and retraining employees on these updated procedures.
- Equipment Upgrade: In the facility, routine testing uncovers a persistent issue with product contamination linked to outdated equipment. The corrective action is to replace or upgrade the faulty equipment to ensure product safety.
Preventive Actions
Preventive actions are proactive steps taken to eliminate the causes of potential non-conformances or other undesirable situations. These actions are intended to prevent issues before they occur, thereby reducing the risk of defects, non-compliance, and other problems.
Examples of Preventive Actions in the Pharmaceutical Industry
- Implementation of Advanced Analytics: Utilizing predictive analytics to anticipate equipment failures before they happen, allowing for maintenance or replacement, thereby preventing potential production disruptions or quality issues.
- Supplier Quality Management: Establishing a comprehensive supplier quality management program to assess and monitor the quality of raw materials, components, and packaging materials to prevent quality issues downstream in the manufacturing process.
- Environmental Monitoring Enhancements: Proactively improving the environmental monitoring programs in manufacturing areas, such as cleanrooms, to detect and mitigate risks of contamination before they affect product quality.
Corrections
Corrections in the context of GMP for pharmaceutical manufacturing refer to immediate actions taken in response to an identified non-conformance or issue, aimed at rectifying the situation at hand without delving into the underlying root causes. These are typically quick fixes to address immediate quality or compliance issues.
Examples of Corrections in Pharmaceutical Manufacturing
- Immediate Batch Quarantine: In response to the detection of a potential quality defect in a batch, the immediate correction is to quarantine the batch to prevent its distribution until further investigation can determine the nature and extent of the issue.
- Equipment Adjustment: If a piece of equipment is found to be operating outside of its specified parameters, a correction would involve adjusting the equipment to bring it back into compliance until a more thorough analysis can be conducted to understand why the deviation occurred.
- Documentation Correction: Correcting a mistake in batch documentation immediately upon discovery to ensure accurate records are maintained, pending a more thorough review to prevent recurrence.
Integrating Corrective Actions, Preventive Actions, and Corrections in GMP
Integrating corrective actions, preventive actions, and corrections within the GMP framework is essential for maintaining high-quality manufacturing practices. Corrections address immediate issues, corrective actions tackle the root causes of specific problems to prevent their recurrence, and preventive actions aim to mitigate potential future risks. This comprehensive approach not only ensures the quality and safety of pharmaceutical products but also fosters a culture of continuous improvement and compliance with regulatory standards.
CAPA Process
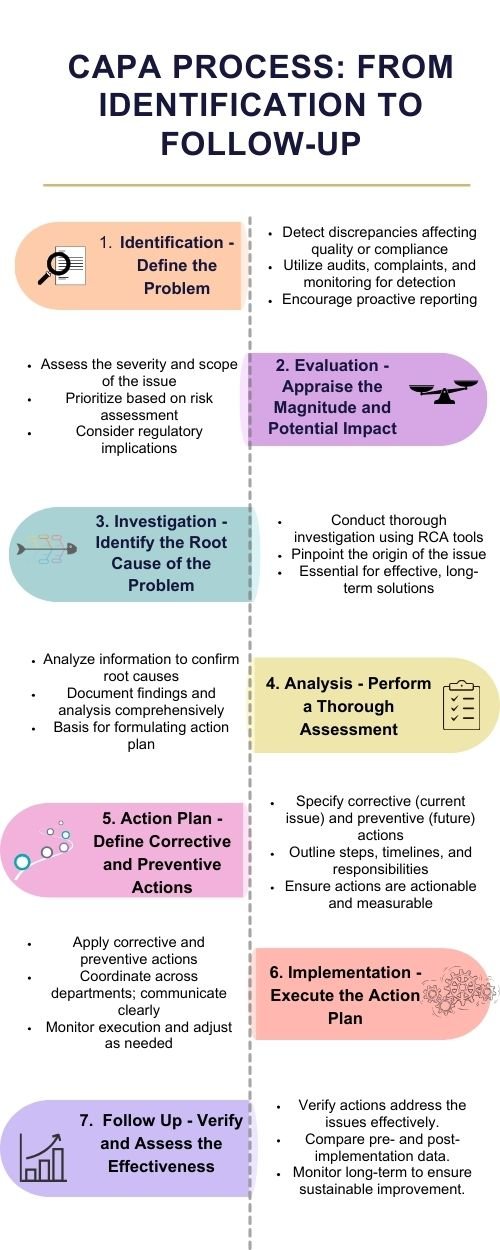
Developing and implementing CAPA plans is a structured process that ensures issues are effectively addressed and prevented from recurring. This process can be summarized into several critical steps:
Identification – Define the Problem
The identification stage is the cornerstone of the CAPA process. It involves the detection of discrepancies, non-conformities, or incidents that potentially impact product quality, safety, or regulatory compliance.
This detection can occur through various channels such as quality audits, customer complaints, internal inspections, process monitoring, and review of process performance indicators. Effective identification relies on a vigilant and proactive quality management system (QMS) that encourages reporting and documentation of potential issues.
Evaluation – Appraise the Magnitude and Potential Impact
Once an issue is identified, it must be promptly evaluated to determine its scope and impact on product quality and patient safety. This step involves assessing the severity of the issue, the extent of affected products or processes, and the potential regulatory implications. The evaluation helps in prioritizing CAPA activities based on risk assessment, ensuring that resources are allocated to address the most critical issues first.
Investigation – Identify the Root Cause of the Problem
The investigation phase is aimed at understanding the underlying reasons for the identified issue. Root cause analysis (RCA) tools such as the 5 Whys, Fishbone Diagrams (Ishikawa), and Failure Mode and Effects Analysis (FMEA) are commonly used to delve deep into the problem and pinpoint its origin. A thorough investigation is essential to ensure that the CAPA not only addresses the symptoms of the problem but eliminates the root causes to prevent recurrence.
Analysis – Perform a Thorough Assessment With Documentation
In this critical phase, the information gathered from the investigation is analyzed to confirm the root causes and understand their mechanisms fully. This analysis is meticulously documented, providing a clear and comprehensive basis for devising an effective action plan. Documentation at this stage includes detailed findings, data analysis, identified root causes, and the rationale behind the conclusions drawn.
Action Plan – Define Corrective and Preventive Actions
With a clear understanding of the root causes, a detailed action plan is formulated. This plan specifies the corrective actions (to address and rectify the current issue) and preventive actions (to mitigate the risk of future occurrences). The plan outlines specific steps, timelines, responsibilities, and resource allocations. The proposed actions must be actionable, measurable, and aligned with the overall objectives of the quality management system.
Implementation – Execute the Action Plan
This stage involves the practical application of the corrective and preventive actions as outlined in the action plan. Effective implementation requires coordination across different departments and functions, clear communication of the plan and its objectives, and adequate training or instruction as necessary. The execution of the plan is closely monitored, with adjustments made as needed to ensure its success.
Follow-Up – Verify and Assess the Effectiveness
The final step in the CAPA process is to verify that the implemented actions have effectively addressed the identified issues without introducing new problems. This involves comparing pre- and post-implementation data to assess the impact of the changes. The effectiveness of the CAPA is monitored over time to ensure that the corrective and preventive actions have resulted in sustainable improvements. If necessary, the process may be repeated to further refine the actions and outcomes.
Executing Effective Root Cause Analysis (RCA)
Executing an effective Root Cause Analysis involves a structured approach to uncover the underlying reasons for nonconformities, ensuring that corrective and preventive actions (CAPA) are accurately targeted. The process can be broken down into several key steps:
Assemble the Right Team
It’s crucial to gather a cross-functional team to minimize bias and avoid inter-organizational politics, ensuring a comprehensive analysis from multiple perspectives. The team should include individuals with relevant expertise and an understanding of the processes and systems in question.
Choose the Appropriate RCA Tool
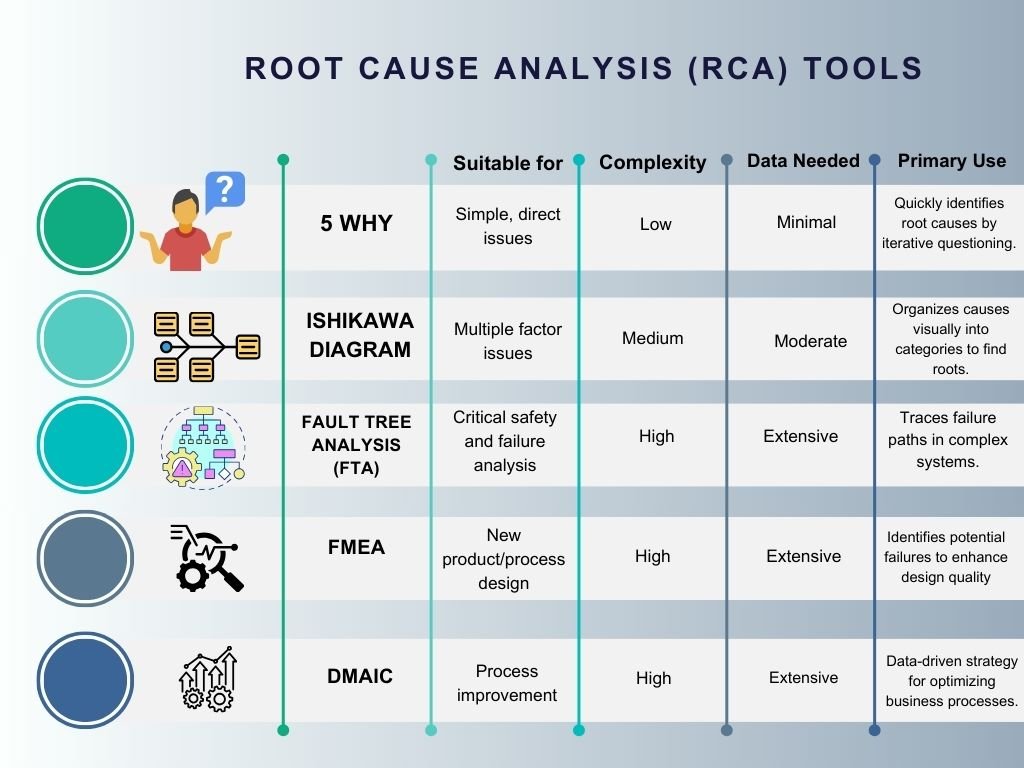
Several tools and methods can be employed depending on the issue at hand, including:
- 5 Whys: A simple technique that involves asking “why” multiple times until the root cause is identified.
- Fishbone Diagram (Ishikawa Diagram): Helps visualize the potential causes of a problem, categorizing them to identify the root cause.
- Fault Tree Analysis (FTA): A deductive method that starts with an adverse event and works backward to determine the root cause.
- Pareto Analysis: Based on the principle that a majority of problems (80%) are often due to a few key causes (20%).
- Failure Modes and Effects Analysis (FMEA): Focuses on identifying potential failure modes and their impact on product/process performance.
- DMAIC (Define, Measure, Analyze, Improve, Control): A data-driven improvement cycle used for enhancing, optimizing, and stabilizing business processes and designs.
The choice of tool should be based on the complexity of the problem, the available data, and the specific outcomes desired.
Conduct the RCA Process
The RCA Process should include the following:
- Investigation and Analysis: Begin with a thorough investigation of the problem, utilizing the chosen RCA tool to dissect the issue systematically.
- Identify Root Causes: Focus on identifying not just the immediate causes but also the underlying systems or processes that contribute to the problem. It’s important to recognize that what might initially appear as human error is often rooted in process or system failures.
- Document Findings: Use an Electronic Quality Management System (eQMS) to document the RCA process and findings. This helps in effectively managing the risk-based CAPA process and connecting RCA outcomes to CAPA actions, ensuring a streamlined workflow and better compliance.
- Develop CAPA Plans: Based on the RCA outcomes, develop CAPA plans that are designed to eliminate and prevent the root causes identified. This step is crucial for ensuring that the same issues do not recur.
Remember, the effectiveness of RCA lies in its ability to dig deep into the problem, going beyond superficial solutions to address the underlying causes. By following a structured approach and utilizing the right tools, organizations can significantly enhance their CAPA process, leading to improved quality, safety, and compliance.
RELATED ARTICLE: 6 Steps on How to Perform RCA
Monitoring and Verifying CAPA Effectiveness
Monitoring and verifying CAPA effectiveness involves a series of strategic steps aimed at ensuring the root causes of nonconformities are not only identified but effectively addressed to prevent recurrence. This process is critical for maintaining GMP compliance and enhancing overall product quality and safety. The following outlines the key components involved in this process:
Verification Steps
- Evaluate Impact and Risk: Assess the potential impact and risk level of the problem to prioritize CAPA activities effectively.
- Verify Actions: Ensure the completion and effectiveness of actions through testing, monitoring, or additional inspections.
- Data Dissemination: Determine if information regarding nonconforming products, quality problems, and CAPA has been properly disseminated for management review.
- Effectiveness Checks: Conduct effectiveness checks to build confidence in the solution, validate that the solution worked, and minimize any risks or potential problems that might cause reoccurrence.
Tools and Methods
- Digital Systems: Utilize digital systems and software for RCA and CAPA management to centralize and automate the recording and tracking of customer complaints, product holds, and deviations.
- Measurement Methods: Employ both qualitative and quantitative methods to measure CAPA success, considering who will measure it, what and where it will be measured, when and how it will be measured, and how measurements will be analyzed.
- Verification Methods: Implement methods such as trend analysis, periodic checks, surprise audits, and sampling to verify CAPA effectiveness.
TIP: Evaluate the effectiveness of a CAPA by meeting deadlines, the effectiveness of containment actions, elimination of the issue by addressing root causes, re-opening of CAPAs if actions are ineffective, and adherence to CAPA processes.
This structured approach to monitoring and verifying CAPA effectiveness ensures that corrective and preventive actions are not only implemented but are also effective in addressing the root causes of nonconformities, thereby supporting continuous improvement and compliance with regulatory requirements.
Common CAPA Problems/Mistakes
Some of the common CAPA mistakes that happen in the GxP environment include:
- Inadequate Root Cause Analysis: A superficial investigation that fails to identify the true underlying causes of an issue can lead to ineffective corrective actions that do not prevent recurrence.
TIP: Invest in training for employees on root cause analysis techniques to ensure that investigations are thorough and effective.
- Poor Documentation and Tracking: Insufficient documentation and tracking of CAPA processes can lead to a lack of accountability and transparency, making it difficult to verify the effectiveness of actions taken.
TIP: Use digital tracking and documentation tools to maintain detailed records of CAPA processes, ensuring accountability and facilitating review.
- Resistance to Change: Organizational resistance to implementing changes suggested by CAPA findings can hinder the improvement process and lead to recurring problems.
TIP: Encourage an organizational culture that views CAPA as an opportunity for improvement rather than a punitive measure, reducing resistance to change.
- Overreliance on Quick Fixes: Prioritizing immediate solutions without addressing systemic issues can result in repeated failures and increased regulatory scrutiny.
TIP: Involve personnel from various departments in the CAPA process to gain diverse perspectives and foster a collaborative approach to problem-solving.
- Lack of Resources: Insufficient allocation of resources, including time, personnel, and budget, can lead to incomplete implementation of CAPA measures.
TIP: Ensure that sufficient resources are allocated to the CAPA process, recognizing that the cost of prevention is often lower than the cost of failure.
FAQ
When Should a CAPA Be Initiated?
A CAPA should be initiated when an issue is identified that affects product quality, and safety, or violates regulatory standards. This can be triggered by customer complaints, internal audits, non-conformities, process deviations, or as a result of regulatory inspections.
How Long Should a CAPA Remain Open?
The duration for which a CAPA remains open depends on the complexity of the issue, the effectiveness of the actions taken, and the verification of their efficacy. It should only be closed once evidence shows that the corrective and preventive measures have been effective and there is confidence that the issue will not recur.
What Documentation Is Required for a CAPA?
Documentation for a CAPA includes the initial report of the problem, the investigation findings, and root cause analysis, the action plan (including who is responsible for each action and timelines), and records of the implementation and effectiveness verification of the actions taken.
Can a CAPA Be Reopened After It Has Been Closed?
Yes, a CAPA can be reopened if the issue recurs or if subsequent monitoring and evaluation indicate that the actions taken were not effective in fully addressing the problem.
Summary – How to Implement CAPA Plan Successfully
The systematic approach to identifying, investigating, and resolving quality issues not only aligns with regulatory standards but also fosters a culture of continuous improvement. This commitment to excellence is evident in the meticulous steps outlined for CAPA development, including the diligent identification of issues, execution of effective root cause analysis, and the strategic development and implementation of corrective and preventive plans.
As we reflect on the significance of CAPA in maintaining high standards, it becomes clear that its success hinges on rigorous monitoring, verification of effectiveness, and ongoing refinement. The potential for CAPA to drive meaningful change within organizations is profound, provided there is a steadfast adherence to the established procedures and an openness to adapting processes based on effectiveness checks.















