Ensuring the reliability, accuracy, and completeness of data is essential for safeguarding public health. Regulatory bodies like the FDA and EMA have stringent guidelines to uphold data integrity, emphasizing its critical role in maintaining drug safety, efficacy, and quality.
This article explores the principles of data integrity, the regulatory framework, and best practices for compliance, providing a comprehensive guide for pharmaceutical professionals to navigate this crucial aspect of GMP.
What Is Data Integrity?
Data integrity means that all data must be accurate, complete, and maintained in its original or true copy form throughout its lifecycle. Accurate and reliable data ensures that drugs are produced consistently and meet quality standards, essential for patient safety. It encompasses the principles of being attributable, legible, contemporaneous, original, and accurate (ALCOA).
Metadata plays a significant role in this context, serving as structured information that describes and makes data easier to retrieve, use, or manage. Without metadata, a data value remains meaningless, lacking the necessary context.
Ensuring data integrity means that any recorded information can be trusted to be true and unchanged, providing a reliable basis for decision-making in manufacturing and controlling pharmaceutical products.
Any compromise in data integrity can lead to serious consequences, including releasing substandard or harmful products, regulatory actions, and loss of public trust.
Key Concepts in Data Integrity
The key concepts in data integrity include:
ALCOA+ Principles
The ALCOA+ principles form the foundation of data integrity, ensuring data is reliable and accurate throughout its lifecycle. The principles are:
- Attributable: Clearly linked to the individual who created it.
- Legible: Easily readable and permanent.
- Contemporaneous: Recorded at the time of the activity.
- Original: The first record or a certified copy.
- Accurate: Free from errors.
Additionally, the data should be:
- Complete: Containing all necessary information.
- Consistent: Following a consistent format.
- Enduring: Available for the required duration.
- Available: Accessible for review and audit.
RELATED: Good Documentation Practices (GDocP)
Metadata
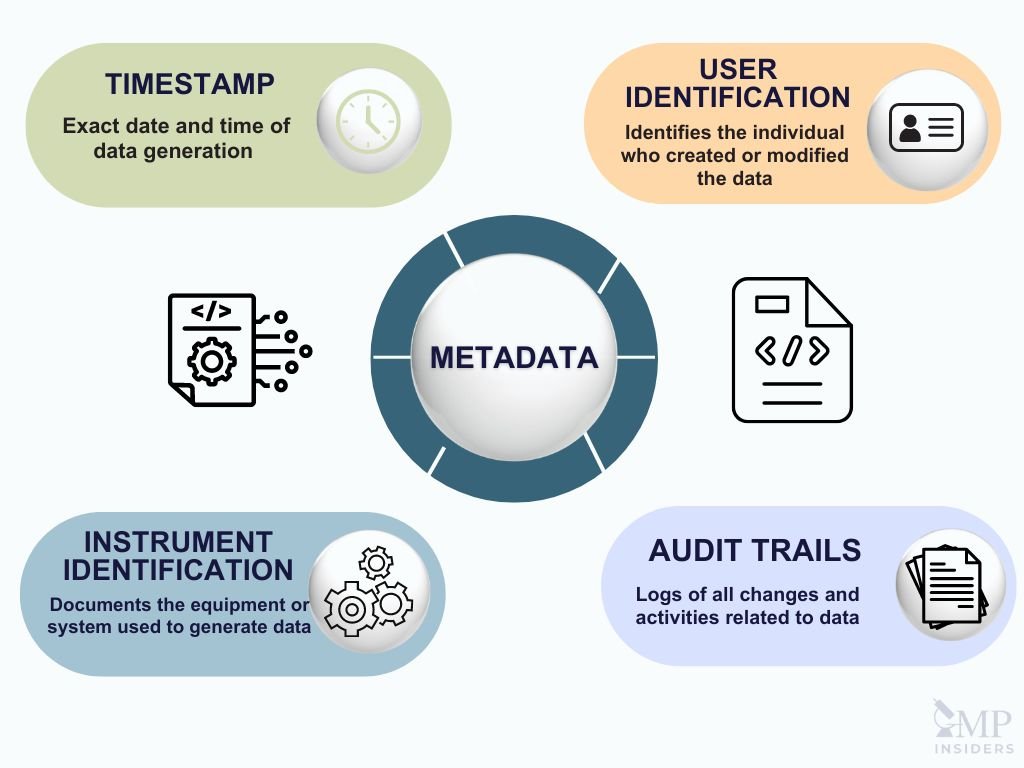
Metadata is structured information that provides details about other data, helping to describe, explain, and facilitate its retrieval and management. In the context of cGMP (Current Good Manufacturing Practices), metadata is essential for ensuring that electronic data is meaningful and reliable. Without metadata, digital data lacks context and cannot be properly interpreted or used. For instance, a numerical value needs associated metadata to define its unit, source, or relevance.
Components of Metadata
Key components of metadata in the cGMP context include:
- Timestamp: Recording the exact date and time when data was generated or modified.
- User Identification: Identifying the individual who created or altered the data, which aids in accountability.
- Instrument Identification: Documenting the equipment or system used to generate the data, ensuring traceability back to the source of the data.
- Audit Trails: Logs that capture all changes and activities related to the data, providing a history of how and by whom the data has been handled.
Why Metadata is Critical
Metadata ensures that data remains complete and interpretable by providing the necessary context. For example, a temperature reading without metadata indicating the unit (Celsius or Fahrenheit), the location of the measurement, or the time it was recorded would be incomplete and potentially misleading.
Audit Trails
An audit trail is a secure, computer-generated, time-stamped record that allows for the reconstruction of events relating to the creation, modification, and deletion of data. The purpose of audit trails is to provide a chronological sequence of activities that can be used to verify the integrity of data and ensure compliance with regulatory requirements.
Audit trails play a crucial role in maintaining data integrity by:
- Tracking Data Creation: Recording when and by whom data was originally generated.
- Monitoring Modifications: Documenting any changes made to the data, including who made the changes and when.
- Recording Deletions: Capturing any deletions of data to ensure that data removal is authorized and traceable.
- Logging Access Attempts: Tracking attempts to access, rename, or delete files, which helps in identifying unauthorized activities.
Best Practices for Implementation and Review
- Enable Audit Trails: Ensure audit trails are enabled for all critical data-related activities and systems.
- Protect Audit Trails: Protect audit trails from unauthorized access, modifications, and deletions.
- Review Regularly: Regularly review audit trails to detect and investigate any anomalies or suspicious activities.
- Training: Train staff on the importance of audit trails and how to interpret and use them.
- Automated Monitoring: Implement automated systems to monitor audit trails and alert relevant personnel to potential issues.
- Documentation: Keep detailed documentation of audit trail procedures and ensure they are part of the organization’s quality management system.
What Is Data Governance?
Data governance is a comprehensive system designed to ensure data integrity throughout its entire lifecycle. This means making sure that data is always accurate, reliable, and accessible, no matter how it’s created, processed, stored, or used.
Implementing a data governance system isn’t legally required, but it helps organizations manage data integrity risks effectively. Without it, data integrity efforts might be fragmented, leading to possible gaps in control.
Data Lifecycle
The data lifecycle refers to all the stages that data goes through, from its creation to its eventual disposal. This includes generating, processing, reporting, verifying, using, storing, and discarding data.
Throughout this lifecycle, data may move between different systems (e.g., from paper to digital) and different departments or organizations, both internally (like production, quality control, and quality assurance) and externally (such as service providers).
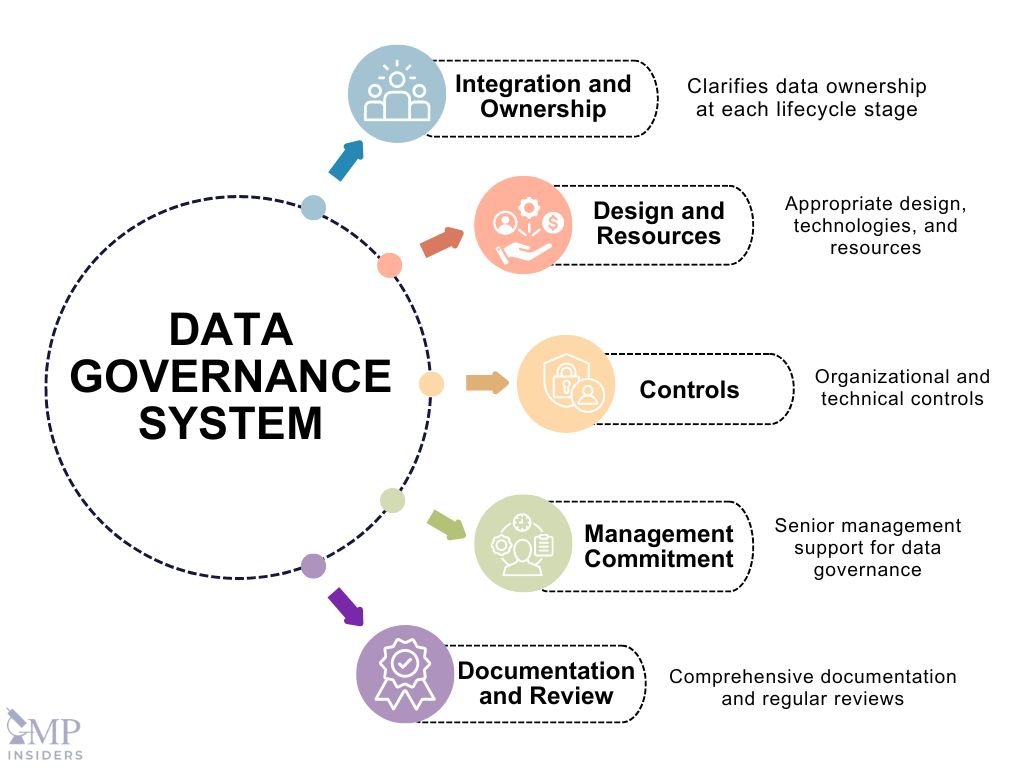
Components of Data Governance Systems
The data governance systems include the following components:
Integration and Ownership
A good data governance system should be part of the overall Pharmaceutical Quality System. It should clarify who owns the data at each stage of its lifecycle and ensure processes and systems are designed to maintain data integrity, including preventing unauthorized changes or deletions.
Design and Resources
For a data governance system to be effective, it needs to be well-designed and use appropriate technologies and security measures. This requires expertise in data management and integrity. Organizations must allocate sufficient resources to design, develop, operate, and monitor these systems, taking into account the complexity of their operations and the importance of the data.
Controls
Controls are essential to maintaining data integrity and should be proportional to the risk involved. These controls can be:
- Organizational: Establishing clear procedures for handling data, training staff, regularly verifying data, conducting self-inspections, and involving experts in data management.
- Technical: Validating computerized systems, using automation to reduce human error, and employing technologies that enhance data security and management.
Management Commitment
Senior management must be committed to data governance, understanding its importance, and fostering a culture that supports data integrity. Employees should feel empowered to report any issues or suggest improvements without fear of reprisal, which helps prevent data falsification or deletion.
Documentation and Review
All data governance practices should be documented and regularly reviewed as part of the Pharmaceutical Quality System. This ensures that they remain effective and up-to-date.
Data Criticality
The significance of data varies depending on the decision it influences and its impact on product quality or safety. For example, data used to make batch release decisions is more critical than warehouse cleaning records. Similarly, assay data for an active pharmaceutical ingredient (API) has a greater impact on product quality and safety than tablet friability data.
Data Risk
All GMP/GDP data must meet data integrity requirements, but assessing data criticality helps prioritize efforts. The rationale for this prioritization should be documented according to quality risk management principles.
Data risk assessments should evaluate the likelihood of data being altered, deleted, lost, or falsified, and how easily such actions can be detected. Measures should be in place to prevent unauthorized activity and increase the visibility of changes.
RELATED: Quality Risk Management
Factors Increasing Data Risk
Complex processes, inconsistent methods, and subjective outcomes increase the risk of data failure. In contrast, simple, well-defined processes with objective tasks reduce risk. Risk assessments should focus on business processes, evaluating data flows, and methods of data generation and processing, rather than just IT system functionality.
Data Governance System Review
The effectiveness of data integrity control measures should be periodically assessed through self-inspections (internal audits) or other review processes to ensure that controls over the data lifecycle are functioning as intended.
In addition to routine data verification checks, self-inspections should include:
- Verifying continued understanding of good data management practices among personnel.
- Reviewing the consistency of reported data against raw entries.
- Sampling computerized system logs or audit trails to ensure accurate reporting of relevant information.
- Analyzing quality system metrics that may indicate the effectiveness of data governance.
An effective review of the data governance system demonstrates an understanding of how company behaviors interact with organizational and technical controls. The results of the review should be communicated to senior management and used to assess any residual data integrity risks.
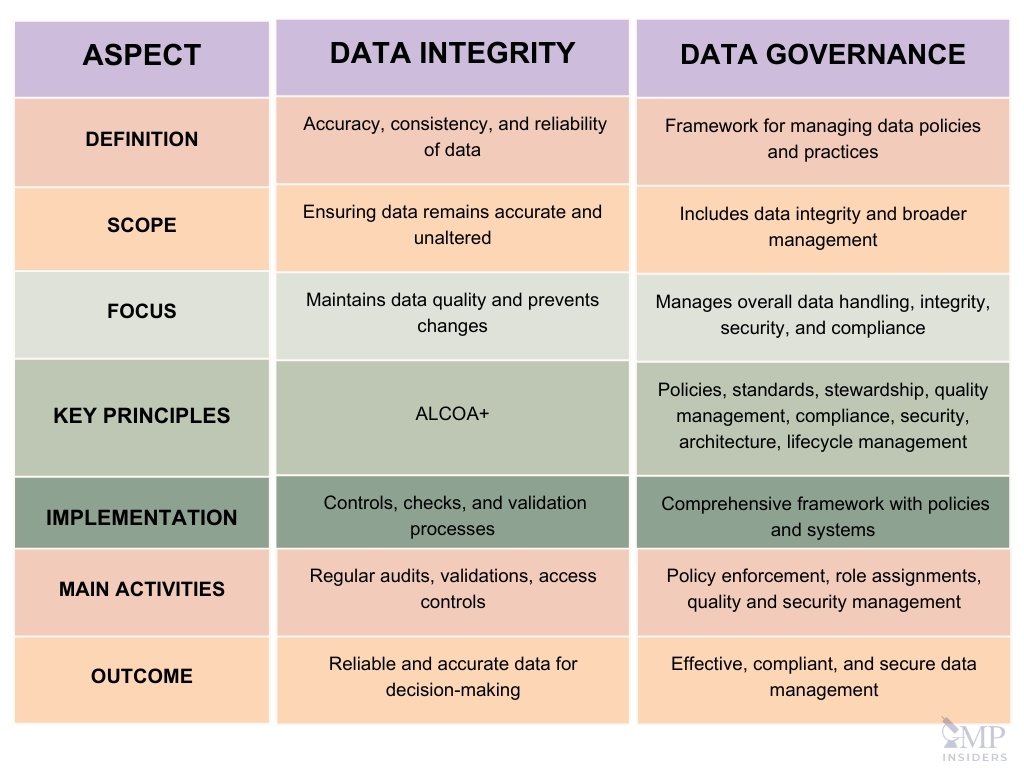
Comparison Between Data Integrity and Data Governance
Specific Data Integrity Considerations for Paper-Based Systems
Effective management of paper-based documents is crucial in GMP/GDP environments. The documentation system must be designed to meet GMP/GDP requirements, ensuring that documents and records are controlled effectively to maintain their integrity. This includes keeping paper records attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and available (ALCOA+ principles) throughout their lifecycle.
SEE ALSO: ALCOA++ in GMP
The documentation procedures should be outlined within the Pharmaceutical Quality System. These procedures must cover the creation, review, and approval of documents; the control of templates and master documents; the processes for retrieval and disaster recovery; the issuance and reconciliation of working copies; and the proper completion and filing of documents.
The generation, distribution, and control of template records require master documents to have unique identifiers and version control, with uncontrolled documents prohibited. Documents must be designed with sufficient space for data entries and updated and distributed in a timely manner. Obsolete versions should be archived appropriately, and document issuance should be strictly controlled.
At the point of use, records must be readily available to operators and protected from damage. Handwritten entries should be clear, contemporaneous, and made in indelible ink to ensure they are enduring. Corrections to records must be made by crossing out errors with a single line, providing an explanation if necessary, and signing and dating the changes.
Verification of records involves an independent review of critical process steps at the time of occurrence and subsequent review by production and quality units. For simple systems like balances and pH meters that generate direct print-outs, original records should be signed, dated, and include necessary traceability information.
Data Integrity Considerations for Computerized Systems
Companies use a variety of computerized systems, from simple standalone to complex integrated ones, impacting product quality. It’s crucial to evaluate and manage these systems according to GMP (Good Manufacturing Practice) and GDP (Good Distribution Practice) standards.
Each system’s use, function, and potential data integrity risks must be assessed. Systems impacting product quality should be managed under a Pharmaceutical Quality System to protect against accidental or deliberate manipulation.
Data Vulnerability and Risk Management
Assess data vulnerability and risk within the business process context. For instance, analytical results depend on various factors, such as sample preparation and data processing. A data flow map can help identify system vulnerabilities. Older systems may need extra protections like firewalls and intrusion detection. Inspections should utilize company expertise to effectively assess systems. Guidelines ensure data integrity within computerized systems, applying equally to outsourced services.
For example, chromatography records need special caution when processed, and data integrity should be maintained by implementing adequate practices to safeguard the integrity of the electronic record.
SEE ALSO: Data Integrity in Chromatographic Peak Integration
Data Transfer and Migration
Validate interfaces between systems to ensure secure data transfer. Use secure transfer methods, encryption, and checksums. Maintain data readability throughout its lifecycle, possibly requiring software preservation or data migration. Control and verify migration to ensure complete data transfer.
System Security for Computerized Systems
User access controls must prevent unauthorized data access, changes, or deletions. Systems should have individual logins and passwords, restrict critical data access, and generate login attempt logs. Implement physical and network security measures like firewalls and intrusion detection to protect systems from threats.
Audit Trails for Computerized Systems
Audit trails must always be enabled, recording changes and deletions of critical data. Regular reviews should focus on changes or anomalies, integrating audit trails into routine data review processes with clear review procedures.
Qualification and Validation of Computerized Systems
Qualification and validation should follow GMP/GDP guidelines. Computer System Validation ensures records are protected but must be supplemented with controls and user training. Documentation should cover data management and integrity controls from procurement to lifecycle management. Maintain an inventory of systems, including their function, criticality, and validation status. Periodic evaluations ensure compliance and detect potential issues.
SEE ALSO: Qualification vs Validation in GMP
Storage, Archival, and Disposal of Electronic Data
Ensure data storage captures all data and relevant metadata using secure, validated processes. Regular backups and secure archival ensure data integrity and accessibility. Disposal procedures must prevent unauthorized access and comply with retention requirements.
Practical Tips for Maintaining Compliance
Maintaining consistency and continuity in data integrity within Good Manufacturing Practices (GMP) is paramount to ensuring that pharmaceutical products meet quality standards consistently. This involves a combination of long-term strategies and overcoming various challenges.
Long-Term Strategies
Establish Robust Data Governance Policies: It is crucial to implement clear and consistent data governance policies that define roles, responsibilities, and rules for data management. These policies should align with GMP regulations and ensure accountability, transparency, and compliance throughout the data lifecycle.
Implement Technical and Procedural Controls: Firms should utilize a mix of technical and procedural controls to maintain and document data integrity. For instance, employing systems like Laboratory Information Management Systems (LIMS) or Electronic Batch Record (EBR) systems that automatically save data after each entry can help in adhering to cGMP documentation practices.
RELATED: Electronic Quality Management Systems (eQMS)
Routine Audit Trail Reviews: Regularly scheduled audit trail reviews are recommended to monitor changes to critical data. This ensures that all modifications are documented and verifiable, adhering to CGMP requirements.
Training and Education: Continual training of personnel on data integrity principles is essential. Ensuring that all staff have the necessary training to perform their duties effectively helps in identifying and mitigating data integrity issues.
Overcoming Challenges
Managing Data Across Its Lifecycle: Data must be managed carefully from creation through archiving. This includes ensuring data completeness, consistency, and accuracy at every stage of the data lifecycle
Preventing Data Breaches and Loss: Implementing data encryption and authentication methods is vital to prevent unauthorized access and data breaches, which can compromise product quality and patient safety.
Utilizing Data Validation and Verification Tools: Employing tools that check data for accuracy, completeness, and consistency helps in detecting and correcting errors or discrepancies that may occur during data collection or analysis.
Adopting Data Integrity by Design: Incorporating data quality and security considerations into every stage of the data lifecycle can prevent data errors or tampering from occurring, rather than relying on corrective actions after the fact.
Conducting Regular Audits: Data security and integrity audits assess the effectiveness and compliance of data management practices. These audits help in monitoring performance, identifying improvement opportunities, and demonstrating adherence to GMP standards.
By implementing these strategies and addressing challenges effectively, pharmaceutical companies can ensure the integrity of their data, thereby maintaining consistency and continuity in product quality and compliance with regulatory standards.
FAQ
What Role Does Employee Training Play in Maintaining Data Integrity?
Employee training is crucial for maintaining data integrity as it ensures that all personnel understand the importance of accurate data entry, proper documentation practices, and compliance with regulatory requirements. Regular training updates keep employees informed about new threats and best practices.
How Can Companies Ensure the Integrity of Data Collected From Third-Party Vendors?
Companies can ensure the integrity of data collected from third-party vendors by establishing clear data integrity requirements in contracts, conducting regular audits of vendor processes, implementing secure data transfer protocols, and requiring vendors to provide detailed documentation and validation of their data management practices.
What Are Some Best Practices for Maintaining Data Integrity in a Cloud-Based System?
Best practices include implementing strong access controls, using encryption for data storage and transmission, ensuring regular data backups, employing robust audit trails, and conducting regular security assessments and compliance audits.
How Does Data Integrity Relate to Cybersecurity?
Data integrity and cybersecurity are closely related as both aim to protect data from unauthorized access, alteration, and loss. Strong cybersecurity measures such as firewalls, intrusion detection systems, encryption, and access controls help ensure that data remains accurate and unaltered, thereby maintaining its integrity.
Conclusion
Ensuring the highest data integrity standards with adherence to ALCOA+ principles, combined with rigorous data governance practices, is essential for maintaining compliance with regulatory bodies such as the FDA and EMA. Accurate, complete, and reliable data not only guarantees product quality but also protects patient safety and upholds public trust.
Pharmaceutical companies must integrate robust data governance systems within their operations, encompassing technical and procedural controls, regular audits, and continuous employee training. These measures ensure that all data is meticulously managed throughout its lifecycle, from creation to archival, and is readily accessible for regulatory review.















