Design reviews (DRs) and design qualification (DQ) are essential processes in the design of new or modified systems. These processes ensure that design deliverables are consistent with user requirements, mitigate potential risks, and comply with regulatory requirements.
DRs ensure that design deliverables align with user requirements and control strategies developed during the system risk assessment. DQ, on the other hand, involves more formal documentation, with the approval of the quality unit, to demonstrate traceability of critical aspects of the design to critical quality attributes.
The Relationship Between Design Review and Design Qualification
Design Review and Design Qualification are closely related activities in the equipment validation process. While they are separate documentation processes, they are interconnected and work together to ensure the quality and suitability of the system design.
DR focuses on both critical and non-critical aspects of the system design, evaluating deliverables against standards and requirements and identifying any issues or gaps that may need to be addressed. The final report from DR serves as a key input into the DQ process.
DQ, on the other hand, is more focused on critical aspects (CAs) and critical design elements (CDEs), which are essential for ensuring product quality and patient safety. It involves formal documentation and approval from the Quality Unit, demonstrating the traceability of these CAs/CDEs to the Critical Process Parameters (CPPs) and Critical Quality Attributes (CQAs).
Read more in our article Qualification vs Validation to learn the key differences.
Table 1: Comparison Table for Design Review (DR) and Design Qualification (DQ)
Aspect | Design Review (DR) | Design Qualification (DQ) |
Purpose | To evaluate design deliverables against standards and user requirements. | To provide documented verification that the design meets all requirements for its intended purpose. |
Key Activities | Evaluating specifications, assessing against standards, and identifying issues or gaps. | Formal documentation and approval, verification of design against quality attributes and standards. |
Documentation Required | Design specifications, evaluation reports, and issue logs. | Quality Unit approval documents and design verification records. |
Stakeholders Involved | Design engineers, quality assurance teams, and project managers. | Quality Unit, regulatory compliance experts, subject matter experts. |
Outcome | A comprehensive report detailing the alignment with standards and identification of any issues. | Formal documentation confirming the design’s suitability for its intended purpose. |
The Design Process
The design process can be divided into three phases: concept, basis of design (BOD), and detailed design. These phases help in developing design deliverables, identifying and mitigating risks, and achieving financial objectives.
Concept Phase: The primary output of this phase is the evaluation of different design approaches to meet the specified user requirements. This involves considering various options and methods that could potentially fulfil the needs of the project.
Based on this evaluation, the most appropriate design approach is selected for further development. This decision is critical as it sets the direction for the entire project.
Basis of Design (BOD): In this phase, the focus is on developing a detailed scope of work. This document expands on the chosen design concept from the Concept Phase, providing a more detailed and comprehensive plan of what the project will entail.
Additional engineering work is carried out to refine and elaborate on the selected concept. This involves turning the initial idea into a more detailed and practical plan, ensuring that all aspects of the design are considered and planned for.
Detailed Design: The output of this final phase is the ‘Issued for Construction’ (IFC) documents. These documents represent the culmination of all previous design work and are essentially the final, detailed plans that will be used to construct or implement the system.
This stage involves detailed engineering and finalizing the design. It’s where all the planning and preparation come together, and the project is brought to a point where it is ready for implementation or construction.
Table 2: Project Phase and Deliverables
Project Phase | Deliverables | Feeds into DR | Feeds into DQ |
Concept | Evaluation of scope options, preliminary design concepts | Initial assessment of design concepts against user requirements | Preliminary alignment with user requirements |
Basis of Design (BOD) | Detailed scope of work, refined design concepts | Review of detailed design plans and compliance with standards | Verification of design elements against quality standards |
Detailed Design | Issued for Construction (IFC) documents, final design specifications | Final evaluation of design specifications for completeness and compliance | Final verification and approval of the design for its intended purpose |
Science and Risk-Based Approach to Design Reviews
A science and risk-based approach is crucial in conducting effective Design Reviews. Quality Risk Assessments (QRAs) of the manufacturing process, including environmental conditions and critical systems, provide valuable insights that influence the early phases of the design process.
Read more about Quality Risk Management in our article.
Quality Risk Assessments (QRAs)
QRAs are a pivotal component of the design review process. They involve a detailed analysis of potential failure modes within the design. These assessments provide critical insights that influence early design decisions, steering the project toward solutions that mitigate risks effectively.
By identifying and understanding potential quality risks at the outset, the design can be tailored to preemptively address these concerns, thereby reducing the likelihood of issues during later stages of development and production.
System Risk Assessments
Complementing QRAs, System Risk Assessments focus on the design concerning process control. These assessments examine how the design interacts with and affects the manufacturing process, identifying risks to process stability and product quality.
The goal is to ensure that all potential risks, whether related to equipment, materials, or process parameters, are thoroughly evaluated and mitigated. This includes considering the impact of design choices on critical process parameters and ultimately on the product’s critical quality attributes.
Design stage System Risk Assessments help evaluate system design about process control failure modes. These assessments are linked to process risk assessments and other process knowledge, ensuring that the design addresses identified risks. Incorporating these assessments into Design Reviews helps identify critical aspects (CAs) and critical design elements (CDEs) necessary for risk control.
Design stage quality risk reviews should be iterative and integrated into the design development process. Reviewing the System Risk Assessments and their impact on the design should be included in the scope of Design Reviews to ensure a comprehensive and effective approach.
Table 3: Example of Risk Assessment Table for the Design Review (DR) process
| Risk | Likelihood | Severity | Mitigation Strategy | Responsible Party |
| Inadequate user requirements specification | Medium | High | Detailed review and validation of user requirements | Project Manager |
| Design not compliant with regulatory standards | High | Very High | Regular updates and training on regulatory changes | Regulatory Affairs Specialist |
| Insufficient testing of design | Medium | High | Comprehensive testing protocols throughout design phase | Quality Assurance Team |
| Inadequate consideration of manufacturing capabilities | Low | Medium | Early involvement of manufacturing team in design process | Design and Manufacturing Engineers |
| Poor integration with existing systems | High | High | Ensuring compatibility and integration testing in early design stages | IT and Systems Integration Team |
Design Review
Design reviews (DRs) are planned and systematic evaluations of specifications, design, and design development throughout the lifecycle of the manufacturing system. The objectives of DRs are to evaluate deliverables against standards and requirements, identify problems, and propose necessary corrective actions. DRs encompass both quality critical and non-critical aspects of the system design.
Objectives of Design Review
Design Review (DR) serves specific objectives in the equipment validation process. According to ASTM E2500-13, DR is a planned and systematic review of specifications, design, and design development carried out throughout the life-cycle of the manufacturing system. The primary objectives of DR include:
- Evaluating deliverables against standards and requirements
- Identifying problems, gaps, and deviations from the URS
- Proposing required corrective actions to address identified issues
By conducting comprehensive Design Reviews, potential design-related issues can be identified and resolved early on, reducing the need for costly change orders and ensuring the system’s compliance with quality and regulatory requirements.
Role of Subject Matter Experts and Quality Unit
The success of the Design Review process relies on the active involvement of Subject Matter Experts (SMEs) and the Quality Unit. SMEs bring their technical expertise and knowledge of the process, equipment, and facilities to ensure that the system design meets the requirements of the User Requirement Specifications (URS).
The Quality Unit plays a crucial role in ensuring that all quality requirements are met during the Design Review process. Their involvement facilitates the review and approval of Design Qualification, simplifying the overall validation process. Collaborating effectively with SMEs and the Quality Unit ensures a comprehensive and successful Design Review process.
Design Review Process
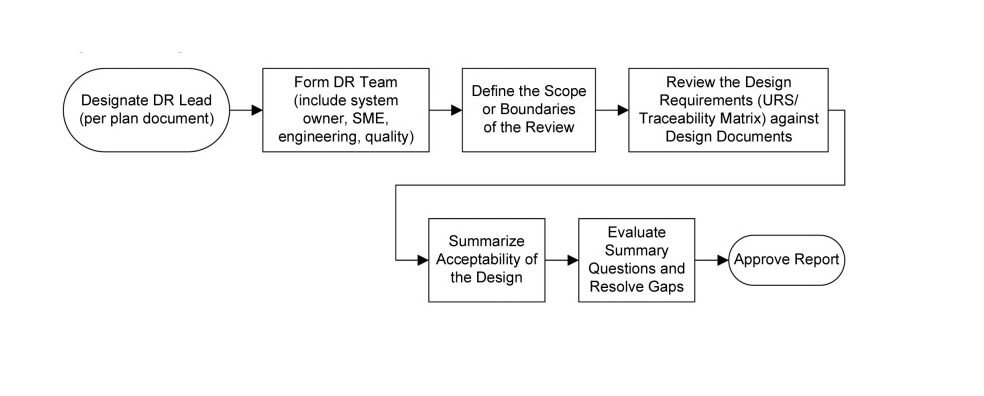
Figure 1: Design Review Process Flow in accordance with ISPE Baseline Guide Vol 5: Commissioning & Qualification 2nd Edition
The Design Review process should be well-planned and executed to ensure a thorough evaluation of the system design. Before the Design Review, design documents should be shared with the project team for their preliminary review. The project manager and design leads are responsible for ensuring the appropriate participants are involved in the Design Review.
During the Design Review, the project team should evaluate the design deliverables, including engineering standards, business requirements, operations requirements, and quality requirements. The review should assess the alignment of these deliverables with the URS and identify any gaps or issues that require corrective actions.
The level of formality and documentation of Design Reviews should be commensurate with the level of risk associated with the system. For highly critical systems, more focused and detailed Design Reviews may be necessary, while off-the-shelf equipment may undergo simpler reviews. The Design Review process should address both engineering design and quality aspects to ensure a robust and compliant system design.
Design Review Outcome
The outcome of the Design Review process should be properly documented and managed to ensure the continuing suitability of the system design. The results of the Design Review should serve as the basis for any necessary design modifications or additions. This documentation should include any identified deficiencies in the design and the corresponding corrective actions taken.
The project team should establish effective methods for recording and distributing the results of the Design Review, as well as managing any changes to the design. The results should be communicated to the respective management levels, ensuring that all stakeholders are aware of any design-related issues and their resolution.
By documenting the outcomes of the Design Review process, organizations can maintain a comprehensive record of the design decisions, changes, and justifications, ensuring transparency and accountability throughout the equipment validation process.
Design Qualification
Design qualification (DQ) is the documented verification that the design of a new or modified direct impact system is suitable for its intended purpose. The objectives of DQ are to verify that the design meets quality user requirements, adequately control risks to product quality and patient safety, and address critical aspects of the design.
Table 4: Design Qualification Criteria
Criteria | Method of Verification | Documentation Required |
Compliance with User Requirements Specification (URS) | Comparison of design specifications with URS | Design specification documents, URS |
Alignment with Quality Standards | Review of compliance documents and quality control tests | Quality compliance certificates, test reports |
Risk Mitigation Effectiveness | Evaluation of risk assessment documentation and mitigation strategies | Risk management reports, mitigation plans |
Integration with Existing Systems | Testing for compatibility and functional integration | Integration test reports, compatibility analyses |
Operational Efficiency and Reliability | Performance testing and reliability assessments | Performance test records, reliability |
Objectives of Design Qualification
The main objectives of design qualification are to ensure that the design incorporates all process and product requirements, addresses quality and regulatory requirements, and verifies the critical aspects necessary for implementing these requirements and risk controls.
Prerequisites
Before conducting design qualification, certain prerequisites must be met. These include an approved user requirements specification (URS), functional requirements for automated systems, system risk assessments with identified critical aspects of the design, and completed design reviews. The project manager is responsible for ensuring that all prerequisites are completed and made available to the participants.
Design Qualification Process
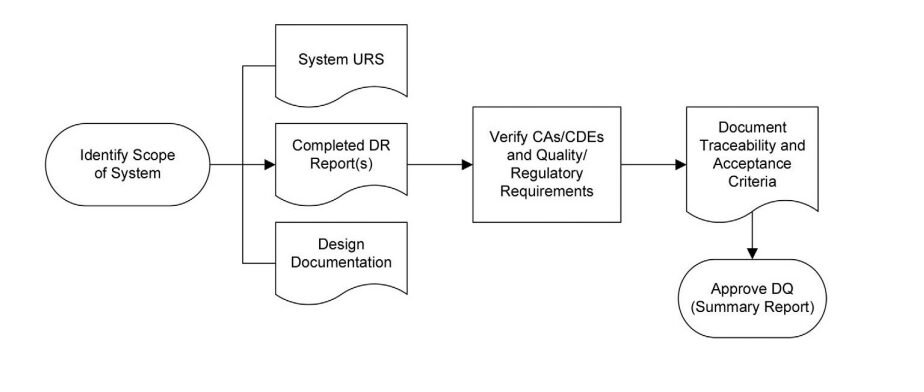
Figure 2: Design Qualification Process Flow in accordance with ISPE Baseline Guide Vol 5: Commissioning & Qualification 2nd Edition
Executing Design Qualification involves following a systematic process and ensuring that all prerequisites are met. The strategy for Design Qualification should be documented in the Commissioning, Qualification, and Validation (CQV) Execution Plan and approved by the Quality Unit.
The prerequisites for Design Qualification include:
- Identification of the correct reviewers and signatories for the DQ
- Approved URS that includes the design controls (CAs/CDEs) necessary to mitigate identified risks
- Completed Design Review(s) that address all open design issues
- Verification of the presence of CAs/CDEs in the proposed design solution
The verification process may vary but should involve mapping each CAs/CDEs point derived from the QRA to objective evidence that confirms their presence in the design solution. The documentation of this verification can take various forms but should ultimately demonstrate that the design is suitable for its intended purpose.
Documentation of Design Qualification
The documentation of Design Qualification should be determined based on the complexity of the project and the strategy adopted. There are several approaches to documenting DQ, and the most appropriate method should be defined in consultation with the Quality Unit and Subject Matter Experts (SMEs).
Three common strategies for documenting DQ include:
- Standalone DQ Document: This approach involves creating a separate DQ document that cross-references the URS quality critical points.
- Integrated Section within the URS: In this approach, the DQ is integrated into the URS itself, with revisions made to include references to design specifications and documentation that confirm the fulfilment of the URS points (CAs/CDEs).
- Integrated Section within the Traceability Matrix: The Traceability Matrix is revised to include the mapping of CAs/CDEs to Critical Process Parameters (CPPs) and subsequent Critical Quality Attributes (CQAs), along with references to the verifications performed.
Regardless of the chosen method, the DQ documentation should explicitly state that the design is suitable for its intended purpose and be approved by representatives from applicable departments and the Quality Unit.
Importance of Good Engineering Practice

Good Engineering Practice (GEP) plays a vital role in the design process and should be followed to ensure the integrity of related data and documentation. During Design Review activities, it is essential to evaluate deliverables against engineering standards, business requirements, and quality requirements while identifying any gaps and proposing necessary corrective actions.
Key Components of GEP
Evaluating Deliverables Against Standards: The first step in GEP involves a rigorous evaluation of design deliverables against established engineering and quality standards. This ensures that every design element meets the stringent requirements of the pharmaceutical industry, from safety to efficacy.
This evaluation is not a one-time activity but an ongoing process throughout the design lifecycle, ensuring continuous alignment with evolving standards and practices.
Identifying Gaps and Implementing Corrective Actions: GEP emphasizes the importance of proactively identifying any gaps or shortcomings in the design process. These gaps could range from minor deviations to significant oversights that could impact the final product’s quality.
Once identified, GEP mandates the formulation and implementation of corrective actions. This approach not only rectifies immediate issues but also contributes to the refinement of processes and standards.
Ensuring Thorough Documentation: Documentation is as critical as the design itself. GEP requires thorough and accurate documentation of all activities, changes, and decisions throughout the design process.
This extensive documentation serves multiple purposes: it provides a traceable record of compliance, supports transparency, and enhances the credibility and reliability of the design.
FAQ
How Is the Effectiveness of Design Reviews and Design Qualifications Measured?
Effectiveness is measured by the degree to which these processes identify and rectify design-related issues, ensure compliance with regulatory standards, and contribute to the timely, cost-effective completion of projects while ensuring product quality and safety.
How Do Changes in Regulations Affect Design Reviews and Qualifications?
Changes in regulations require updates in the Design Review and Qualification processes to ensure ongoing compliance. This may involve revisiting existing designs, updating risk assessments, and revalidating certain aspects of the design.
What Are the Main Objectives of Design Qualification?
The primary objectives of DQ are to confirm that the system’s design fulfils user and quality requirements, effectively controls risks to product quality and patient safety, and addresses critical aspects of the design necessary for implementing requirements and risk controls.
Conclusion
Design Qualification is a vital step in ensuring the suitability and compliance of equipment designs in the pharmaceutical industry. By following a systematic approach to Design Review and Design Qualification, organizations can identify and address potential issues early on, mitigating risks and ensuring product quality and patient safety.
The active involvement of stakeholders, including Subject Matter Experts and the Quality Unit, is crucial for a successful Design Qualification process. By adhering to Good Engineering Practice and incorporating a science and risk-based approach, organizations can enhance the effectiveness and efficiency of equipment validation efforts.















