Every quality decision in GMP manufacturing is only as strong as the data behind it. When that data falls outside approved limits, deviates from an established trend, or shows an anomaly not previously observed, it signals the need for structured investigation.
Such events are first evaluated as potential Out-of-Specification (OOS) or Out-of-Trend (OOT) results. Following investigation, they may be classified as Out-of-Expectation (OOE) if the outcome reflects a previously unrecognized process behaviour that warrants additional review and preventive measures.
The distinction between these classifications defines how promptly action is required, the depth of the investigation, and the potential regulatory impact.
This article explains the definitions and relationships between OOS, OOT, and OOE results, focusing on how each outcome develops during investigation and how they should be addressed within a compliant quality system.
Out of Specification (OOS) Results
An OOS result is a test outcome that does not meet the predefined acceptance criteria listed in regulatory filings (e.g., MAAs, ANDAs, DMFs), official compendia (USP, Ph. Eur., JP), or internal specifications approved by Quality. Any such result is treated as a potential quality defect and must be investigated immediately under a structured and scientifically justified process.
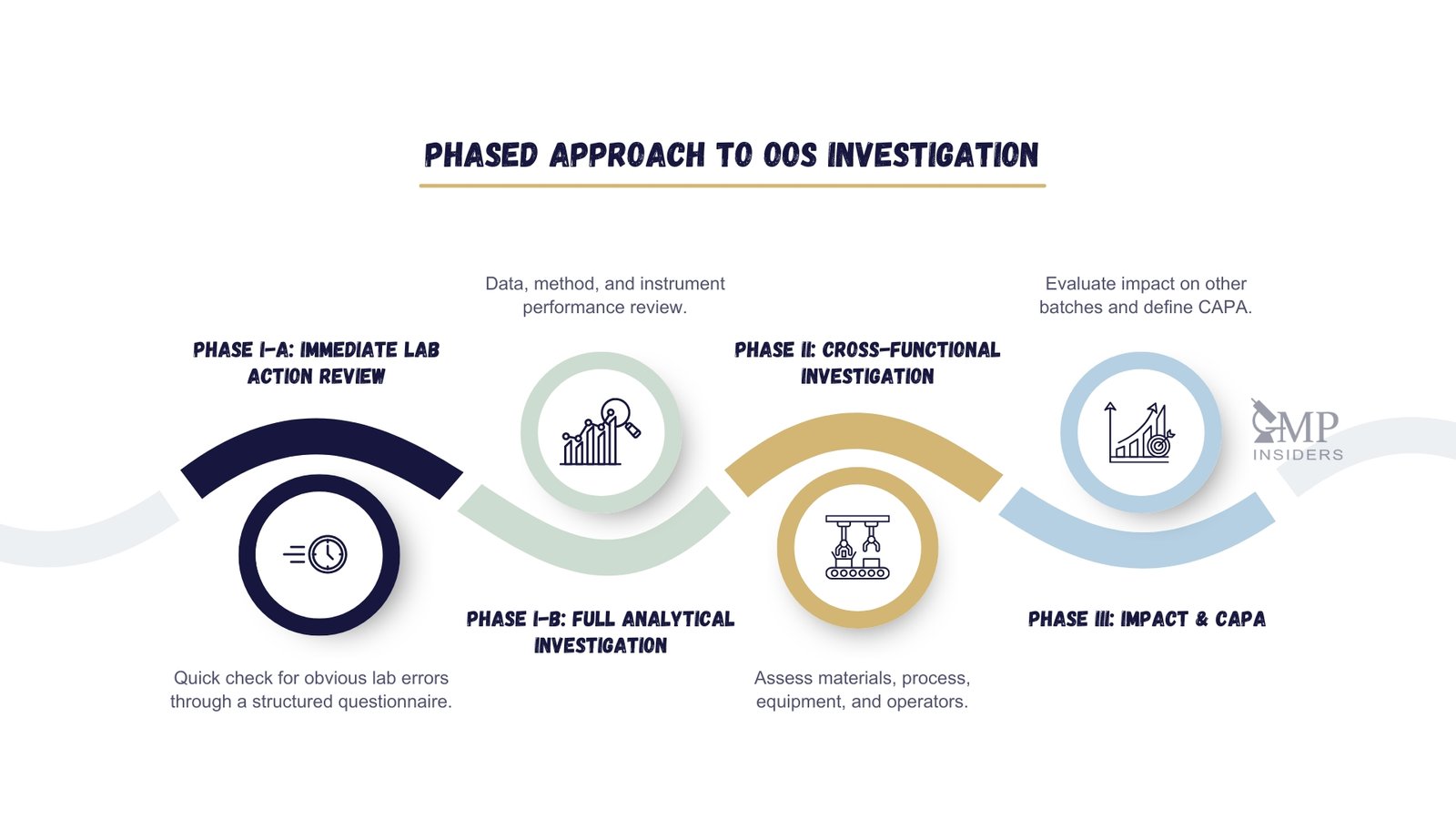
Modern regulatory expectations on OOS (FDA OOS Guidance, EU GMP Chapter 6, MHRA Inspectorate) recognise multiple investigation stages. While FDA guidance refers simply to Phase I (laboratory investigation) and Phase II (manufacturing investigation), the MHRA and many EU-based quality systems further distinguish these into Phase I-A, I-B, II, and III for greater procedural clarity:
- Phase I-A — Immediate laboratory review
Rapid assessment using a structured questionnaire or checklist to detect any obvious laboratory error like transcription mistakes, incorrect solution preparation, reference standard issues, or system suitability failure — performed jointly by the original analyst and the supervisor. - Phase I-B — Full analytical investigation
Deeper review of analytical execution, raw data, instrument performance, and potential assignable lab causes. Repeat testing may occur under controlled conditions if scientifically justified (formal hypothesis must be established). - Phase II — Extended manufacturing/process investigation
Triggered when no laboratory-related root cause has yet been identified. Conducted in parallel with QA-coordinated cross-functional investigation to assess potential process-related causes, including raw materials, equipment, environment, operator execution, or automation/system failure. - Phase III — Impact assessment and CAPA
Required even if the batch is rejected. Evaluates potential impact on other batches, stability studies, or validated processes, and defines long-term CAPA and trending actions.
Investigations into OOS results must be executed without delay, thoroughly documented, scientifically justified, and free from bias or “testing into compliance”.
Corrective and Preventive Actions (CAPA) are expected to be implemented to address the confirmed root cause and prevent recurrence — unless a scientifically justified rationale is documented for not initiating one.
OOS Key Aspects:
- Results fail to meet approved product specifications.
- Trigger immediate investigation upon detection.
- Investigation is structured in phases.
- CAPA implementation is mandatory to prevent recurrence.
Out of Trend (OOT) Results
An OOT result is within the approved specification but deviates from the expected pattern based on historical data, process capability, or stability trends. It often acts as an early warning for emerging product quality or process stability issues.
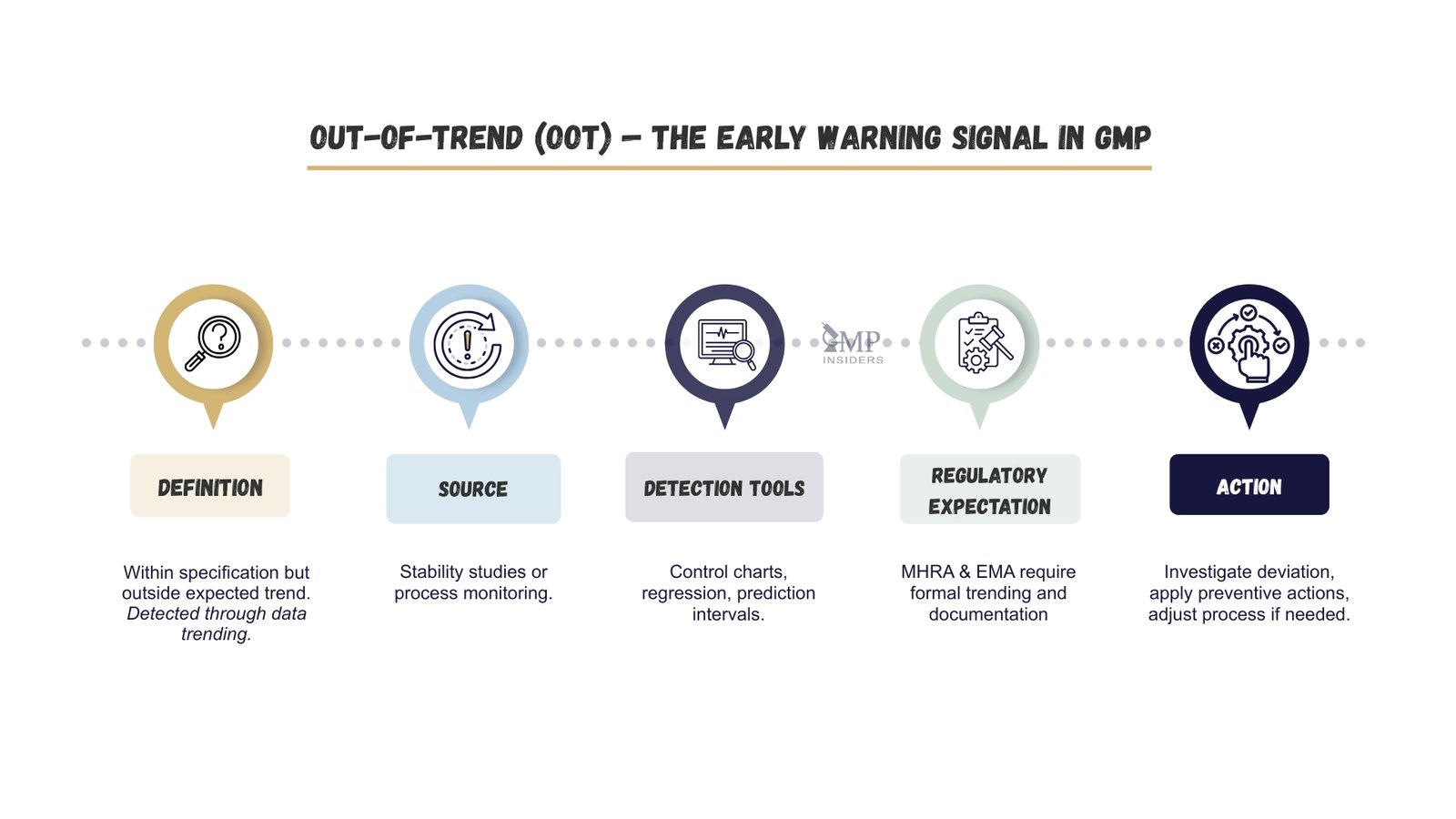
OOT is most commonly detected during:
- Ongoing stability studies – where a parameter drifts but remains within limits (per ICH Q1E and EMA CPMP/QWP/122/02 Rev. 1).
- Process monitoring – where statistical control limits are breached despite meeting specification limits.
MHRA and EMA expect documented procedures for OOT detection and investigation, with statistical tools such as control charts, regression models, or prediction intervals used to confirm deviation significance. Early detection allows for preventive actions, increased monitoring, or targeted process adjustments to avoid escalation to an OOS event.
When such deviations reveal behaviour not previously observed in the process, they may later be characterized as Out-of-Expectation (OOE) results following detailed investigation.
OOT Key Aspects:
- Results are within specification but deviate from expected or historical patterns.
- Often identified through statistical trend analysis.
- Serves as an early indicator of potential quality or stability concerns.
- Requires documented detection and investigation procedures.
- Trend analysis is crucial in the continuous improvement of processes and product quality over time.
Out of Expectation (OOE) Results
An OOE result refers to data that behaves unexpectedly when compared with normal scientific, historical, or statistical behaviour, regardless of whether it is within approved specification limits. Such results are usually identified during or after investigation of an OOS or OOT event, or after targeted verification of a novel within-specification observation that cannot yet be classed as OOT (no trend) or OOS (no limit breach).
There is no harmonised regulatory definition for OOE results in FDA, EMA, or MHRA guidance. However, industry practice, including the ECA Analytical QC Working Group, recognises OOE as an internal signal used to document previously unobserved or scientifically uncharacteristic process behaviour.
OOE investigations focus on determining whether the anomaly represents a first-time occurrence or a known variation. If the investigation of an OOS or OOT result confirms a previously unobserved or unexplained process behaviour, the finding may also be additionally characterised as OOE. When confirmed as a true first-time event, additional preventive actions may be initiated to strengthen process understanding and control.
OOE Key Aspects:
- Determined during or after investigation of unexpected testing results, when the behaviour cannot be explained by known variability.
- Represents a first-time or previously uncharacteristic process response.
- Used internally to enhance process knowledge and early detection capabilities.
- May trigger preventive CAPA or closer monitoring if confirmed as a true first occurrence.
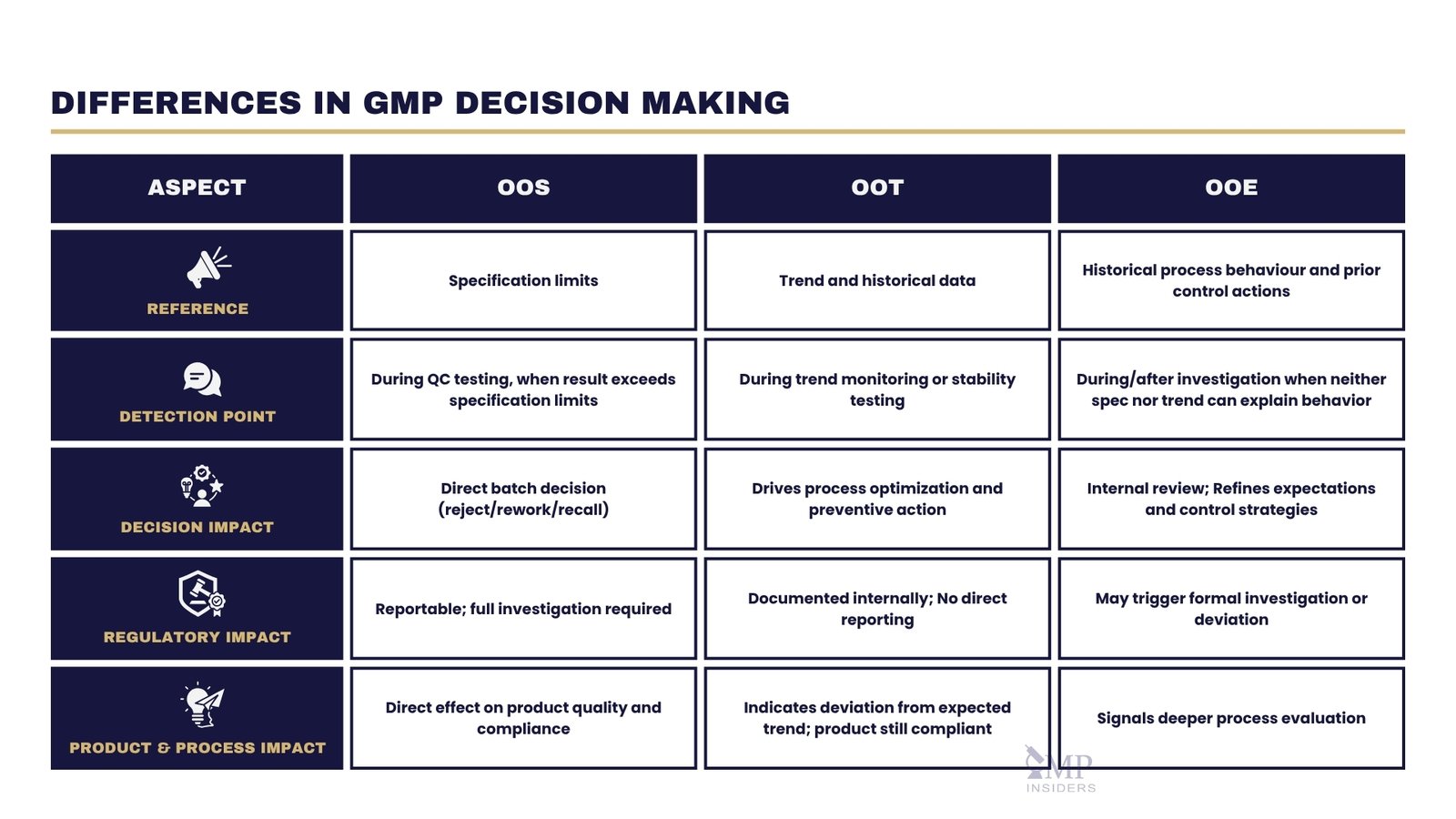
Key Differences Between OOS, OOT and OOE
| Aspect | OOS | OOT | OOE |
|---|---|---|---|
| Reference for Assessment | Specification limits | Trend and historical data | Historical process behaviour and prior control actions |
| Detection Point | During QC testing, when the result exceeds the specification limits | Usually during trend monitoring or stability testing | During/after investigation, when neither spec nor trend can explain behaviour |
| Decision Impact | Direct batch decision (reject/rework/recall) | Drives process optimization and preventive action | Refines expectations and control strategies – internal review |
| Regulatory Impact | Reportable; full investigation required | Documented internally; no direct reporting | May trigger a formal investigation or deviation |
| Product & Process Impact | Direct effect on product quality and compliance | Indicates deviation from the expected trend; product is still compliant | Signals deeper process evaluation |
The following distinctions highlight how OOS, OOT, and OOE results each play a unique role in shaping QC practices, decision-making processes, and overall product quality management:
Reference for Assessment
OOS: Evaluated against approved specification limits defined in regulatory filings or internal quality standards.
OOT: Evaluated against trend data and historical process performance to determine statistical deviation from expected behaviour.
OOE: Evaluated against historical process behaviour and prior control actions when neither specification limits nor trend data explain the observation.
Detection Point
OOS results are typically detected during routine QC testing at release or in-process control, when a measured value exceeds predefined specification limits.
OOT results are identified earlier in the process, often before any specification limit is breached. They are detected through statistical evaluation of historical data, trend charts, control limits, or stability study trends. Detection occurs when a result deviates from the expected pattern or historical mean, even though it remains within specification.
OOE results are raised when neither specification limits (OOS) nor statistical trends (OOT) can explain the unusual observation for certain results. They represent a first-time, uncharacteristic, or scientifically unexplained behaviour observed during a deeper review.
Impact on Decision-Making
OOS results have a direct and immediate influence on product disposition. They can lead to batch rejection, rework, or recall, and almost always trigger prompt corrective actions.
OOT results primarily inform process optimisation strategies. While they rarely require immediate action on a product, they can drive significant long-term process improvements.
OOE results are identified during or after investigation findings when the behaviour represents a previously unobserved or scientifically unexplained response. They guide internal review and refinement of control strategies rather than triggering product-level decisions.
Regulatory Impact
OOS results carry substantial regulatory implications. They require thorough, well-documented investigations and, when confirmed, formal reporting to authorities such as the FDA, EMA, or MHRA. A confirmed OOS can lead to batch rejection or recall.
OOT results generally do not require direct reporting but must be documented and addressed following internal procedures and regulatory expectations to prevent escalation to OOS.
OOE results are not subject to direct regulatory reporting but should be documented internally under site procedures to support decision-making and demonstrate proactive process understanding.
Impact on Product and Process
OOS results directly affect the batch or product in question, often signalling a defect that could compromise safety, efficacy, or compliance.
OOT results may reveal process variability or drift that requires correction, though the product remains within specification at the time of detection.
OOE results signal the need for scientific attention and deeper process evaluation. They rarely require product action but can drive improvements to monitoring strategies and internal control systems.
Practical Examples
While definitions and key differences outline the theoretical framework, real-world cases illustrate how OOS, OOT, and OOE events occur, are investigated, and are resolved in practice. The following examples reflect typical GMP scenarios and demonstrate the decision-making process from detection through to corrective action.
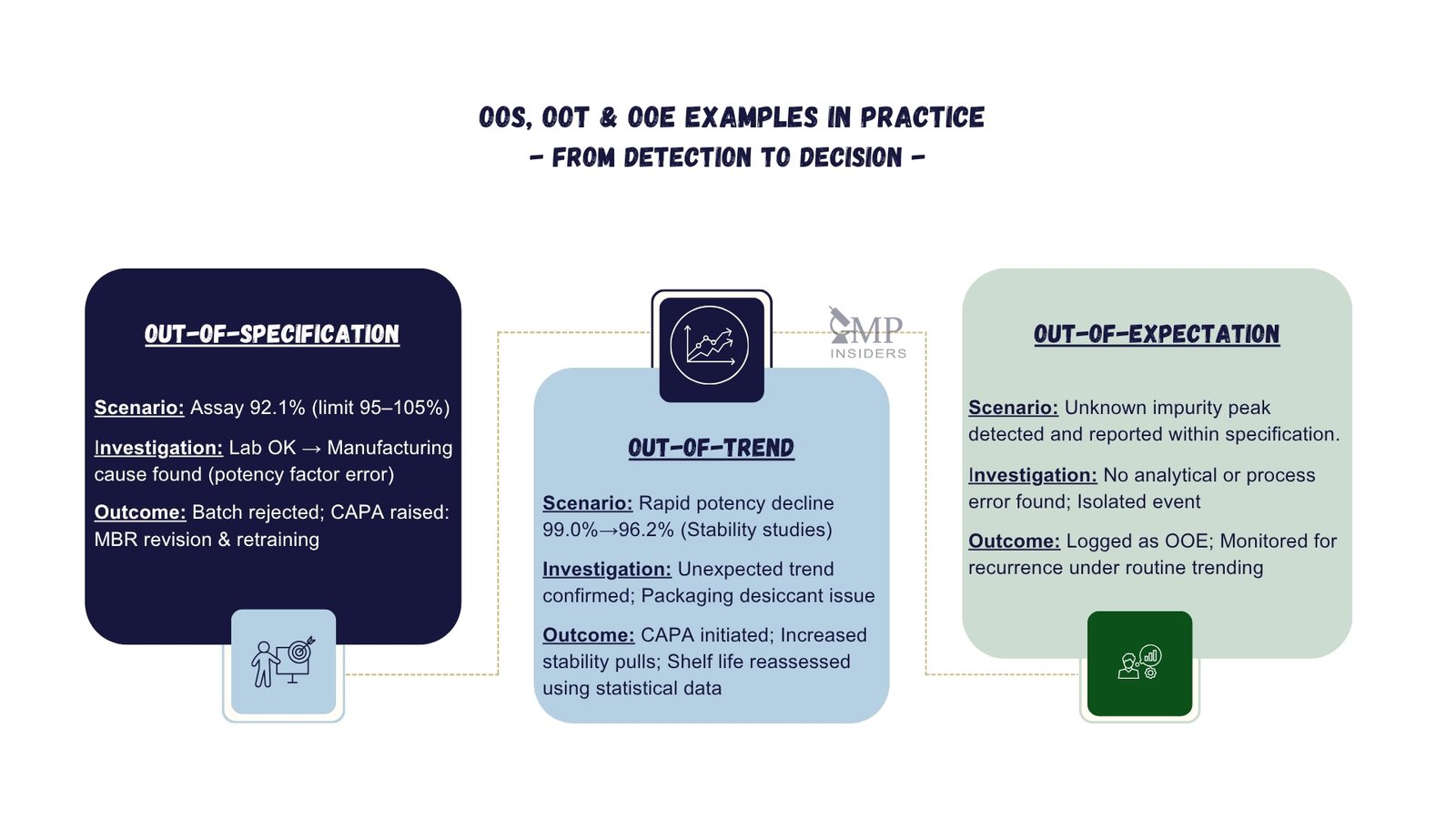
Out-of-Specification (OOS) – Assay Failure at Batch Release
- Scenario: During final QC release testing, a batch records an assay result of 92.1%, below the approved specification range of 95.0–105.0%.
- Investigation: The Phase I laboratory investigation confirms correct method execution, no analytical or procedural errors, and proper instrument calibration. As no lab error is found, Phase II manufacturing investigation is initiated. This identifies that the API potency correction factor was not applied during batch calculation, leading to underdosing of the active ingredient.
- Outcome: The batch is rejected. CAPA includes revision of the master batch record to enforce potency correction checks, retraining of production personnel, and implementation of a second-person verification step before batch calculation approval.
Out-of-Trend (OOT) – Stability Data Drift
- Scenario: A 12-month stability study shows a gradual decline in assay values from 99.0% to 96.2%. Although still within specification, the results breach the internal action limits set for trend monitoring.
- Investigation: Statistical analysis (regression and prediction interval review) confirms a significant downward trend. The investigation links the deviation to inconsistent desiccant loading in the packaging process, potentially affecting moisture control and accelerating degradation.
- Outcome: Corrective actions include revising the packaging SOP to standardise desiccant loading, increasing in-process checks to verify compliance, and adding interim stability pulls to confirm the trend has stabilised.
Out-of-Expectation (OOE) – Isolated Unknown Impurity
- Scenario: During routine HPLC impurities testing, an unidentified impurity peak appears at a new retention time not previously observed for the product. The impurity is below the reportable threshold and within specification limits.
- Investigation: A targeted review confirms correct method execution, validated chromatographic conditions, and no instrument malfunction or sample handling deviations. No known degradant or carryover matches the chromatographic signature of the impurity. Because the result remains within specification (no OOS) and there is no prior pattern to establish a trend (no OOT), the event is handled under OOE verification to confirm a novel, unexplained behaviour.
- Outcome: The finding is characterised as OOE and documented under site procedures. No immediate product impact is identified. The case is monitored for recurrence and for any change in magnitude. Preventive actions (e.g., targeted checks or method robustness review) may be initiated to strengthen monitoring.
Common Causes of OOS, OOT, and OOE Results
Understanding why these events occur is critical for implementing effective controls. While the underlying causes vary, they generally fall into laboratory-related, manufacturing-related, or data-related categories.
Laboratory-Related Causes
- Analytical method issues: Poorly validated methods, inadequate system suitability checks, or inappropriate reference standards can produce inaccurate or inconsistent results.
- Equipment performance problems: Instruments that are out of calibration, poorly maintained, or not qualified can cause erroneous readings.
- Sample handling errors: Incorrect sampling techniques, mishandling, contamination, or degradation during storage can compromise the integrity of results.
- Operator errors: Inadequate training and/or performance, failure to follow SOPs, or data transcription mistakes can introduce avoidable variability.
Manufacturing-Related Causes
- Process parameter deviations: Incorrect temperature, mixing time, granulation endpoint, or compression force can lead to product non-conformance or drift.
- Raw material variability: Changes in supplier, lot-to-lot variability, or near-out-of-spec raw materials can affect final product results.
- Environmental conditions: Uncontrolled humidity, temperature fluctuations, or particulate contamination can contribute to instability or drift.
- Equipment malfunctions: Poorly maintained or uncalibrated manufacturing equipment can introduce inconsistencies in the process.
Data-Related and Statistical Causes
- Data integrity failures: Missing, altered, or incomplete records can mask true results or trends.
- Inadequate trending systems: Lack of robust statistical monitoring allows gradual drifts (OOT) to go unnoticed.
- Statistical misinterpretation: Misuse of control limits, incorrect application of prediction intervals, or ignoring outliers without justification can result in false conclusions.
Regulatory Requirements for Handling OOS, OOT, and OOE Results
Regulators require that any result falling outside expected norms is investigated, documented, and acted upon in a controlled, scientifically justified manner.
While OOS is explicitly covered in guidance documents, OOT expectations are embedded in stability and trend-analysis requirements, and OOE expectations are indirectly reflected through data integrity and investigation principles addressing unexplained or first-time events.
FDA
- Primary Guidance: Guidance for Industry: Investigating Out-of-Specification (OOS) Test Results for Pharmaceutical Production.
- Requires a phased investigation — starting with laboratory checks, followed by a manufacturing review if no lab error is identified.
- Emphasises thorough documentation, root cause identification, and CAPA implementation.
- Warns against “testing into compliance” and stresses that complete investigation records must support product release decisions.
European Union (EU GMP)
- Chapter 6 (Quality Control): Mandates prompt investigation of any result outside specifications or expected norms.
- Requires trend analysis for ongoing stability studies and process data to detect OOT before it escalates to OOS.
- Investigations must be scientifically sound, impartial, and fully documented.
- Product release can only occur after the investigation is completed and justified.
MHRA
- MHRA’s Guidance on OOS aligns with FDA and EU GMP expectations but places additional emphasis on timeliness and data integrity in investigations.
- It requires that OOS, OOT, and other atypical results are not ignored or rationalised without evidence.
- Inspectorate blogs and guidance highlight that OOT investigations should be formalised in SOPs and consistently applied.
ICH and EMA Stability Guidelines
- ICH Q1E: Sets the statistical framework for stability data evaluation, which underpins OOT detection.
- EMA’s Guidance on Stability Testing (CPMP/QWP/122/02 Rev.1): Requires pre-defined OOT limits, documented trending, and escalation procedures.
- Supports early detection of shifts in stability profiles to maintain product quality over shelf life.
FAQ
How Does an OOT Result Differ From an OOS Regarding Process Implications?
An OOT result refers to a finding that deviates from established trends in historical data while within the specification limits. OOT results primarily affect process optimization and may signal the need for preventive measures to avoid future OOS situations.
Can an OOT Result Become an OOS Result?
Yes. If an OOT trend continues and eventually crosses the approved specification limit, it becomes an OOS. For example, a stability assay trend that declines over time but stays in-spec is OOT; if the next time point breaches the limit, it is OOS.
This transition underscores why OOT investigations are critical; they provide an opportunity to act before a compliance failure occurs. Regulatory agencies view ignored OOTs as a missed preventive control.
What Are the Key Regulatory Considerations for OOS, OOT, and OOE Results?
OOS results have significant regulatory implications, requiring detailed reporting and investigation. OOT results may not demand immediate regulatory action but indicate the need for process adjustments.
OOE results, while generally having no direct regulatory reporting requirement, must still be investigated and documented under the site’s quality system to demonstrate control over previously uncharacterised or unexplained data behaviour.
How Often OOS, OOT, and OOE Results Occur, and What Are Their Predictive Values?
OOS results are relatively infrequent but significant when they occur. OOT results can be more frequent, as they are related to trend deviations rather than fixed limits. OOE results are unpredictable and sporadic, often identified through routine data reviews.
What Kind of Investigation Is Typically Required for OOS Results?
OOS results demand a formal and multiphase structured investigation process, including retesting and a detailed root cause analysis to understand why the result was out of specification.
Can OOS, OOT, and OOE Apply to Microbiological Testing?
Absolutely. Examples include microbial counts above limits (OOS), a gradual increase in counts over time (OOT), or a single unusually high count that remains within limits and cannot be attributed to known sources of variation (OOE). Microbial data should be trended and investigated with the same rigor as chemical test data.
Can a Single Failed Replicate in a Multi-Replicate Test be an OOS?
It depends on the acceptance criteria and test method. If specifications apply to the mean of replicates and the mean passes, a single failing replicate may not be classified as OOS. However, it may still be treated as OOE and investigated. For critical quality attributes, even individual replicate failures can trigger heightened review.
What Is the Typical Response to an OOT Result in Pharmaceutical QC?
In response to an OOT result, the focus is on trend analysis and implementing preventive actions to maintain process control and avoid potential future OOS outcomes.
How Do You Handle OOE Results in Method Development?
During method development, OOE findings, first-time or scientifically unexplained behaviours, can guide method robustness improvements. Although they carry no regulatory weight at this stage, documenting them supports lifecycle understanding and prevents recurrence once the method is validated.
Can Method Variability Cause False OOS Results?
Yes. If a method has poor precision or robustness, natural variability may cause occasional failures even when the product is acceptable. This risk highlights the importance of proper method validation and ongoing verification. Part of an OOS investigation involves assessing whether method variability is contributing to the result.
Final Thoughts
Clear distinction between OOS, OOT, and OOE results is more than a matter of terminology; it defines how investigations are prioritised, how risks are managed, and how regulatory obligations are met.
OOE represents the organisation’s ability to recognise and learn from truly unexpected data behaviour, even when no formal specification or trend deviation exists. Misclassification can lead to delayed corrective actions, overlooked process issues, or unnecessary regulatory exposure.
A robust quality system integrates clear definitions, documented investigation procedures, statistical tools for trend analysis, and effective CAPA processes. Equally important is a culture where data anomalies, regardless of their immediate impact, are recognised as opportunities for improvement.
When OOS, OOT, and OOE are managed with consistency, transparency, and scientific rigour, they become tools not just for compliance but for safeguarding product quality and patient safety over the long term.















