The European Directorate for the Quality of Medicines & HealthCare (EDQM) has recently published updated versions of three important water monographs in the European Pharmacopoeia (Ph.Eur.): Water for Injection (0169), Purified Water (0008), and Total Organic Carbon (TOC) in Pharmaceutical Water (2.2.44). These revisions are currently open for public comment.
While the updates are largely editorial, they include some key clarifications:
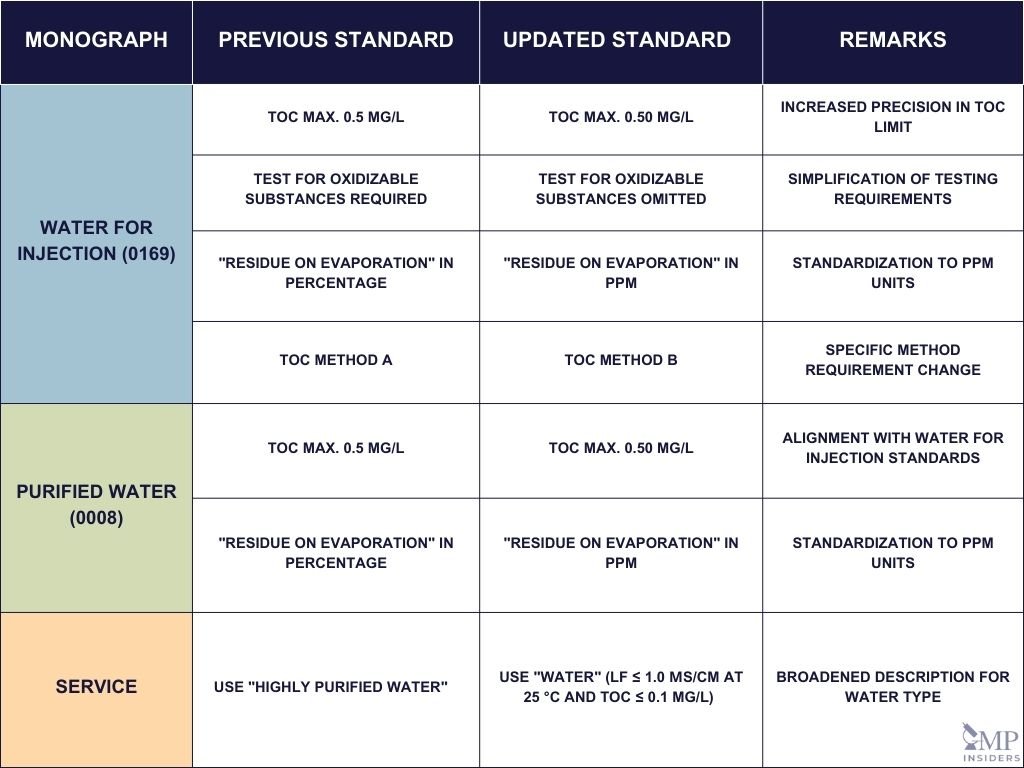
Water for Injection (0169)
TOC Limit Adjustment: To reduce ambiguity, the upper limit for Total Organic Carbon (TOC) has been specified more precisely, changing from 0.5 mg/L to 0.50 mg/L.
Testing Requirements: The requirement to test for oxidizable substances in “Sterilized Water for Injection” has been removed, with TOC method B now mandated for use. This change simplifies the testing procedure by focusing solely on TOC measurements.
Residue on Evaporation: The “Residue on Evaporation” test, which measures non-volatile residue, has been updated to use parts per million (ppm) rather than percentage, offering a more standardized expression of the results.
Purified Water (0008)
Harmonization with WFI Standards: The TOC limit for Purified Water has also been aligned with the update to Water for Injection, now set at a precise 0.50 mg/L.
Standardized Units: For “Purified Water in Containers,” the measurement unit for “Residue on Evaporation” has shifted from percentage to ppm, standardizing the way results are reported.
Total Organic Carbon in Water for Pharmaceutical Use (2.2.44)
Clarification of Water Specifications: The term “highly purified water” has been replaced with “water,” specified to have a conductivity (LF) of ≤ 1.0 µS/cm at 25 °C and a TOC level of ≤ 0.1 mg/L. This change aims to broaden the applicability and clarify the quality required for water used in TOC testing.
These draft revisions aim to improve clarity and precision in the standards for pharmaceutical water, ensuring consistency and reliability in water quality testing. The drafts are available on the European Pharmacopoeia’s website, where they can be reviewed without charge, although a one-time registration is required.
Stakeholders are encouraged to review and provide feedback on these drafts by September 30, 2024. This feedback period offers an important opportunity for professionals in the pharmaceutical and healthcare industries to influence the final standards, ensuring they meet practical and regulatory needs.
For more details and to access the drafts, please visit the European Pharmacopoeia’s official website. Your participation is essential in refining these standards to maintain the highest quality in pharmaceutical water.















