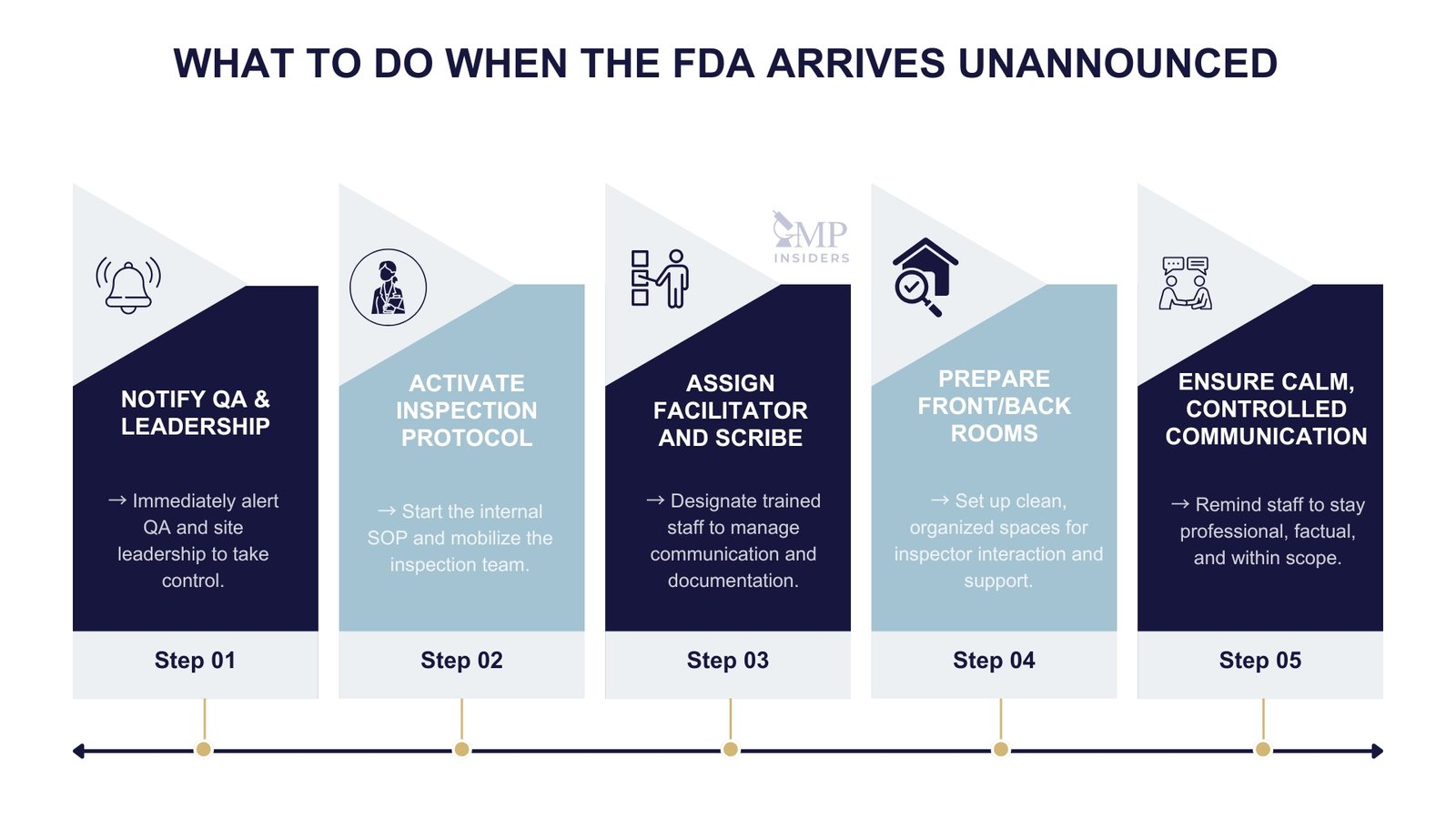
With the U.S. Food and Drug Administration expanding the scope and frequency of unannounced inspections outside the United States, companies worldwide must ensure that their facilities, systems, and personnel are always audit-ready.
Inspections may occur at short notice or without prior warning, and failure to demonstrate a controlled, compliant state can result in significant regulatory action, including Form 483 observations, Warning Letters, import alerts, or delayed product approvals.
This article provides a structured approach to achieving and sustaining FDA inspection readiness. It outlines current expectations, differentiates between inspection types, and offers practical strategies for managing personnel, documentation, facility standards, and inspection conduct.
Understanding FDA Inspections
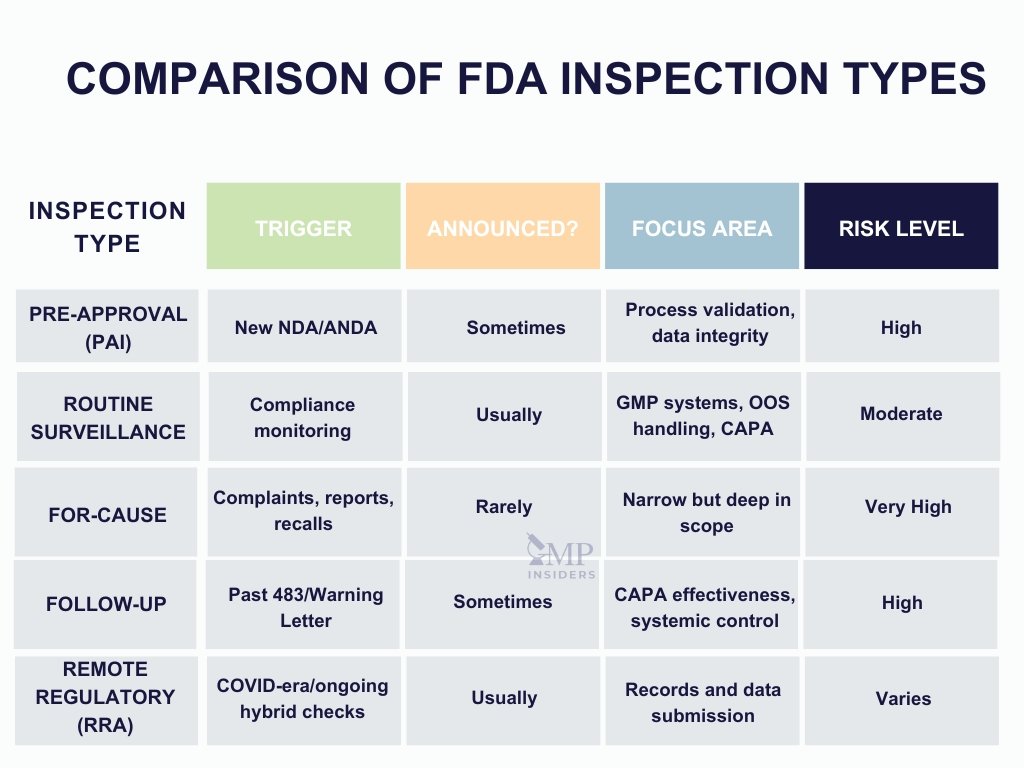
To effectively prepare for an FDA inspection, it is essential to understand the types of inspections the agency conducts and the specific objectives associated with each. The scope, tone, and documentation requests vary depending on the purpose of the inspection and the site’s compliance history.
Pre-Approval Inspection (PAI)
A Pre-Approval Inspection is conducted before the FDA approves a New Drug Application (NDA) or Abbreviated New Drug Application (ANDA). The primary goals are to:
- Verify that the facility is capable of manufacturing the product according to the proposed process
- Confirm that the analytical methods are validated and reproducible
- Ensure that the data submitted in the application reflects actual practices at the site
- Assess data integrity and GMP compliance at the commercial readiness level
These inspections are typically high-risk and highly detailed, particularly for sites that are new to the FDA or have not been inspected recently.
Routine Surveillance Inspection
These inspections are conducted periodically to assess ongoing compliance with 21 CFR Parts 210 and 211 (or applicable regulations for biologics and devices). Key focus areas include:
- Quality systems
- Manufacturing controls
- Laboratory data and OOS/OOT handling
- CAPA effectiveness
- Training and documentation practices
Surveillance inspections are often scheduled but can also be unannounced, especially for sites deemed higher-risk due to past findings or product complexity.
For-Cause Inspection
A for-cause inspection is triggered by a specific concern or risk signal, such as:
- Product complaints or adverse events
- Whistleblower reports
- Import alerts or prior inspection deficiencies
- Recalls or market withdrawals
These inspections are typically unannounced and narrow in scope, but they are often the most intense in terms of scrutiny and the number of documentation requests.
Follow-Up Inspection
Follow-up inspections assess the adequacy and implementation of corrective actions taken after a previous inspection that resulted in significant findings (e.g., a Form 483 or Warning Letter). The FDA will evaluate whether CAPAs have been effectively implemented and whether systemic improvements have been made.
Remote Regulatory Assessment (RRA)
Introduced during the COVID-19 pandemic and increasingly used in hybrid models, RRAs involve the remote review of records and data, often supported by digital submission platforms. While not technically inspections, they may lead to on-site follow-ups or enforcement action if deficiencies are identified.
Building a State of Continuous Readiness
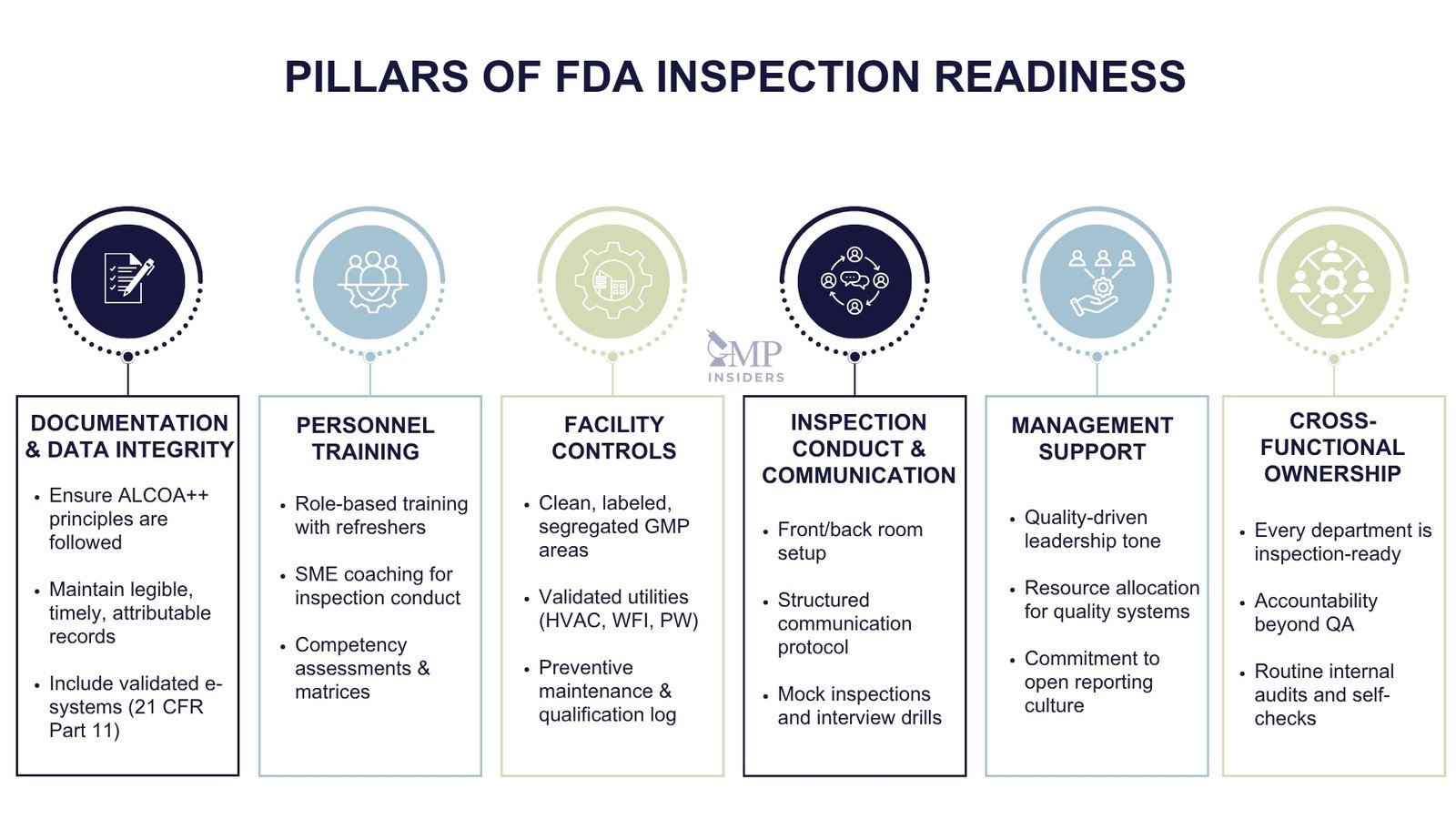
Inspection readiness cannot be treated as a project or event-driven activity. Instead, it must be embedded into the day-to-day operations of the organization. A truly inspection-ready site operates under the assumption that an FDA investigator could arrive at any time, and that systems, documentation, and personnel must consistently reflect a state of compliance.
Integrating Readiness Into Daily Operations
A key principle of continuous readiness is ensuring that GMP activities are documented in real-time, accurately reflect the actual operations, and are readily accessible. This includes:
- Documenting manufacturing, testing, cleaning, and equipment activities as they occur
- Ensuring that logbooks, batch records, and electronic systems are up to date
- Performing routine self-inspections to detect and correct issues proactively
The goal is not to stage compliance, but to live it operationally, so that clean, compliant records and confident staff can validate at any moment in time.
SEE ALSO: Good Documentation Practices (GDocP)
Role of Management and Quality Culture
Leadership sets the tone for readiness. Senior management must demonstrate:
- Visible commitment to GMP compliance
- Resource allocation for training, documentation, and quality systems
- Accountability through quality performance metrics
A compliance-driven culture encourages open communication, continuous improvement, and responsible deviation reporting—all of which contribute to a favorable inspection outcome.
Cross-Functional Ownership
Inspection readiness is not the sole responsibility of the Quality Unit. Every department, manufacturing, warehouse, QC, engineering, and IT must be aware of its role and be prepared to demonstrate ownership of its processes.
This requires:
- Clear communication of expectations
- Periodic inspection-readiness drills or mock inspections
- Consistent enforcement of procedural compliance across all teams
Companies that invest in building this mindset reduce the need for last-minute preparation and lower their risk of inspection-related findings.
Personnel Preparation and SME Training
The conduct, knowledge, and communication of personnel during an FDA inspection are often as critical as the systems themselves. Inspectors will not only review documents and records, but they will also speak directly with operators, analysts, supervisors, and Subject Matter Experts (SMEs) to assess whether the quality system is understood and effectively implemented.
Ensuring Role-Based Training and Competence
The FDA expects each individual to be:
- Trained for their role, with documentation of qualifications and periodic reassessments
- Aware of current SOPs, quality expectations, and the potential impact of their activities on product quality
- Able to explain why a procedure is followed, not just how
Training programs must go beyond procedural reading. They should include:
- Hands-on qualification
- Scenario-based training (e.g., how to handle deviations, data entry errors)
- Periodic knowledge checks to verify understanding
All training must be documented, with training matrices regularly reviewed and updated to reflect organizational changes.
Preparing Subject Matter Experts (SMEs)
SMEs are often the most visible representatives of the company during an inspection. Their readiness can significantly influence the inspector’s perception of control and competence.
Effective SMEs:
- Understand their process end-to-end, including relevant regulatory requirements
- Are able to explain procedures, justifications, and changes confidently
- Know how to navigate documentation and reference data efficiently
- Are trained on inspection conduct, including what to say, when to pause, and how to escalate appropriately
Conducting mock interviews with SMEs in advance helps improve clarity, confidence, and alignment across departments.
SEE ALSO: How to Prepare for a GMP Inspection
Inspection Communication Protocol
Personnel should be trained to follow a structured communication approach during inspections:
- Answer only what is asked, factually and briefly
- Avoid speculation or interpretation—refer to SOPs or escalate if unsure
- Maintain a respectful, professional tone at all times
- Defer to the designated SME or QA representative when questions exceed their scope
Inconsistent answers, visible uncertainty, or over-explaining can raise concerns about the effectiveness of training and internal oversight.
Documentation and Data Integrity
For the FDA, documentation serves as the foundation upon which product quality and Good Manufacturing Practice (GMP) compliance are evaluated. If an activity is not recorded clearly and contemporaneously, it is considered not to have occurred. Likewise, if data integrity cannot be demonstrated, even accurate records may be deemed unreliable.
FDA Expectations for Documentation
All GMP-related documentation—whether paper-based or electronic—must meet the fundamental criteria of completeness, accuracy, traceability, and real-time recording. Inspectors often begin their assessment by reviewing:
- Batch manufacturing production records (BMRs)
- Equipment logs and cleaning records
- Laboratory notebooks and analytical results
- Deviation reports and CAPA documentation
- Training records and change control logs
Documents must be:
- Legible and free of alterations without documented justification
- Signed and dated by the person performing and reviewing the activity
- Consistent with actual operations, including the correct use of SOPs
- Organized and retrievable without delay
Any missing signatures, unexplained corrections, or backdated entries can trigger further questioning and potential observations.
ALCOA++ and Data Integrity Principles
The FDA and global regulators now universally apply ALCOA++ principles to assess data integrity. Data must be:
- Attributable – Who performed the activity?
- Legible – Is the record readable and permanent?
- Contemporaneous – Was it recorded at the time of the activity?
- Original – Is it the first record or a verified copy?
- Accurate – Is the data complete and truthful?
Extended expectations include:
- Complete – All data, including failed or repeat tests, is retained
- Consistent – Time-stamped, chronological, and traceable
- Enduring – Stored securely and protected from degradation
- Available – Readily accessible throughout the retention period
- Accessible and Secure – Protected from unauthorized modification or deletion
- Traceable – Any changes must be documented and audit-trailed
Violations of ALCOA++ principles are among the most frequent causes of FDA 483 observations and Warning Letters, particularly when related to laboratory or electronic data.
21 CFR Part 11 and Electronic Records
For companies using electronic systems, FDA compliance with 21 CFR Part 11 is mandatory. This includes:
- Validated systems, with documented user requirement specifications and qualification
- Audit trails that cannot be disabled and track all changes to data
- Unique user credentials and restricted access
- Electronic signatures that are legally binding and secure
Systems such as LIMS, CDS, MES, and eQMS platforms must demonstrate not only functionality but also integrity and control over the data they manage.
Facility and System Controls
The FDA evaluates not only the documentation and processes at a site, but also the physical environment in which those processes are carried out. The condition of the facility, status of equipment, and effectiveness of environmental and utility controls serve as direct indicators of operational discipline and quality oversight.
Facility Readiness
A compliant facility demonstrates:
- Controlled material and personnel flow to prevent cross-contamination
- Clearly labeled areas, with appropriate status indicators (e.g., “Cleaned,” “In Use,” “Quarantined”)
- Proper segregation of raw materials, intermediates, and finished products
- Access control to restricted manufacturing and QC areas
- Adequate environmental monitoring in accordance with risk-based classifications
The FDA will assess whether the facility layout supports GMP compliance and whether any design weaknesses may compromise product quality. Poor housekeeping, unlabeled containers, or disorganized storage areas can quickly erode confidence in the site’s overall control.
Equipment Qualification and Maintenance
All GMP-relevant equipment must be:
- Qualified before use (IQ/OQ/PQ) with documented protocols and reports
- Subject to routine calibration and preventive maintenance
- Clearly labeled with calibration status and due dates
- Included in an equipment inventory list with usage logs and cleaning records
Any use of unqualified, overdue, or improperly maintained equipment is a common source of FDA 483 observations. The agency often requests a sample of equipment records during inspections to assess whether procedures are being followed consistently and whether deviations are properly investigated.
Environmental and Utility Systems
Critical support systems, such as HVAC, water systems, compressed gases, and cleanrooms, must be validated and controlled to ensure they do not introduce contamination risks.
Key considerations include:
- HEPA filter integrity testing (for classified areas)
- Differential pressure monitoring between rooms
- Routine cleaning and disinfection schedules, supported by logs and monitoring data
- Water for Injection (WFI) and Purified Water (PW) systems are validated and monitored for microbial and chemical quality
- Alarm management and trending for temperature, humidity, and pressure parameters
Failure to maintain these systems or demonstrate ongoing control may lead to findings under process control and contamination prevention expectations.
Mock Inspections and Internal Audits
Routine internal audits and well-structured mock inspections are key tools in preparing for an FDA inspection. They not only help assess the current state of compliance but also reinforce a culture of accountability, encourage cross-functional ownership, and highlight areas requiring corrective action before they become regulatory findings.
Conducting Effective Internal Audits
Internal audits should be conducted according to a formal audit schedule, based on risk level, process criticality, and compliance history. These audits must:
- Be performed by trained, independent personnel
- Follow FDA requirements (21 CFR Parts 210/211 or Part 820, as applicable)
- Include objective evidence collection, not assumptions
- Generate factual, actionable audit reports with traceable findings
- Feed directly into the CAPA system, with timelines and effectiveness checks
Sites should not treat internal audits as checkbox exercises. The most effective audit programs integrate trend analysis, root cause evaluations, and cross-functional feedback loops to support continuous improvement.
SEE ALSO: Root Cause Analysis
Planning and Executing Mock FDA Inspections
Mock inspections simulate a real FDA audit and should replicate:
- The structure of an FDA inspection (including front room/back room setup)
- The type of inspection expected (PAI, surveillance, for-cause)
- Inspector behavior, including documentation requests, walkthroughs, and SME interviews
Ideally, mock inspections should be conducted by external experts with experience in regulatory inspections. This ensures a realistic tone, unbiased evaluation, and exposure to inspection-style questioning.
Key benefits:
- Identifies hidden risks or inconsistencies in documentation
- Tests the responsiveness of SMEs and operational staff
- Validates the inspection management process (e.g., tracking document requests, handling escalations)
- Improves team coordination and response under real-time pressure
Front Room / Back Room Setup for FDA Audits
During an FDA inspection, the organization and control of information flow are critical. Many pharmaceutical companies employ a “front room/back room” model to manage inspections efficiently, minimize delays, and ensure consistent communication.
The Front Room: Managing Inspector Interactions
The front room is where FDA investigators are hosted. It serves as the formal setting for presenting documentation, conducting interviews, and managing the daily inspection agenda. It should be:
- Quiet, professional, and free from operational distractions
- Equipped with essentials: site master file, SOP index, facility map, org chart, contact lists
- Staffed by trained representatives, including:
- A primary inspection coordinator/facilitator
- Relevant Subject Matter Experts (SMEs)
- A scribe to log requests and discussions in real time
Responsibilities in the front room include:
- Receiving and recording document requests
- Responding to questions clearly and within scope
- Coordinating walk-throughs and facility access
- Maintaining a consistent tone and professional conduct throughout the inspection
The Back Room: Inspection Response and Document Management Hub
The back room operates behind the scenes and supports the front room by:
- Locating, reviewing, and preparing requested documentation
- Verifying accuracy, version control, and relevance before submission
- Preparing supporting data (e.g., audit trails, certificates, batch records)
- Drafting clarification responses or compiling data summaries when needed
The back room should include:
- Access to document control systems (eQMS, LIMS, CDS)
- Personnel familiar with site documentation and navigation
- A request tracker to log time, status, and the person responsible for each item
- Communication tools (e.g., phone, chat, shared files) to stay connected with the front room
Why This Setup Matters
The front/back room model:
- Prevents delays and document confusion during the inspection
- Reduces the risk of submitting incorrect or unverified records
- Supports consistent messaging, ensuring only authorized and accurate information is shared with investigators
- Enables faster escalation of complex questions or sensitive issues to the right experts
Even in smaller organizations or virtual inspections, the same principles apply: designate a core team, control document flow, and rehearse roles in advance.
Pre-Inspection Strategy (When Advance Notice Is Given)
Although many FDA inspections are now unannounced, there are still cases where advance notice is provided, particularly for Pre-Approval Inspections (PAIs) or reinspections.
When this occurs, the site should immediately initiate a structured preparation plan to align internal resources, ensure documentation is up to date, and reinforce inspection conduct expectations across departments.
Immediate Steps Upon Notification
Upon receiving the inspection notice:
- Appoint a lead inspection coordinator, typically from Quality Assurance
- Convene a cross-functional inspection readiness team, including SMEs from manufacturing, QC, validation, regulatory, engineering, and IT
- Review the scope of the inspection: application-related, routine, or for-cause
- Notify senior management and prepare internal communication protocols
Documentation Review and Alignment
Start by ensuring that high-risk, high-visibility documents are accurate, current, and aligned with actual practices. Priority documents include:
- Site Master File
- Validation Master Plan (VMP) and major validation protocols/reports
- Organizational chart and training matrices
- Batch production and testing records for recent and application-related products
- Deviation logs, CAPA reports, and change control registers
- Audit trail reviews and access logs for electronic systems
All supporting documentation should be organized by system and easily retrievable by the back room team.
During the FDA Inspection
How an organization conducts itself during an FDA inspection reflects the strength of its quality culture. Even if systems are compliant, poor communication, disorganized documentation flow, or unprepared personnel can negatively influence the outcome. A structured, composed approach throughout the inspection is essential to demonstrate a state of control.
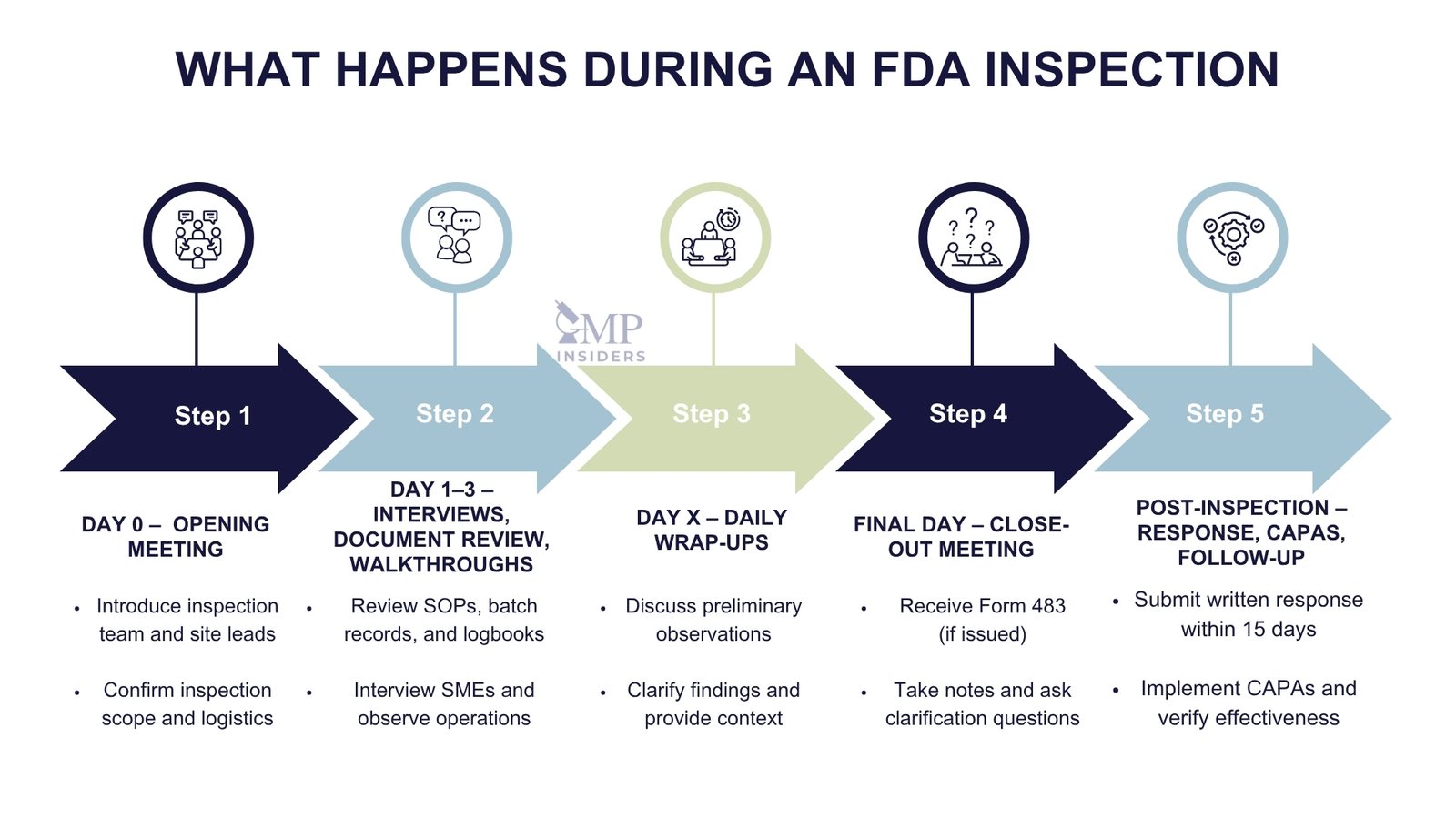
Opening Meeting
The inspection begins with a formal opening meeting, typically led by the FDA investigator. During this session:
- Introduce the inspection team and outline roles (e.g., facilitator, SMEs, scribe)
- Confirm the scope of the inspection and products or applications under review
- Present key documents: Site Master File, organization chart, facility map, product list
- Establish communication protocols for document requests and facility access
Maintain a confident, cooperative tone without over-explaining or offering unsolicited information.
Managing Document Requests
Throughout the inspection, investigators will request documents for review and analysis. The front room team should:
- Log every request with time, description, and response deadline
- Submit only current, approved, and verified versions from the back room
- Provide documents promptly, neatly, and in the order requested
- Avoid excessive background materials unless specifically asked
Never alter documents under inspection or attempt to recreate missing records. If an issue is identified, acknowledge it professionally and offer context through the QA representative.
Facility Walkthroughs
FDA investigators will often request walkthroughs of production, warehouse, or laboratory areas. Escorts should:
- Guide the inspector efficiently and safely
- Ensure areas are clean, labeled, and compliant (e.g., calibration stickers, access control, gowning compliance)
- Be ready to explain room functions, material flow, and critical controls
- Monitor any observations made and relay to the back room for follow-up
Unescorted access or unsatisfactory walkthroughs may indicate broader systemic weaknesses.
Daily Wrap-Ups and Observations
At the end of each day, the FDA investigator may provide informal feedback or clarify points of interest. Use this opportunity to:
- Document potential concerns
- Begin assembling justifications, records, or corrective measures as needed
- Maintain a log of all discussions for continuity and post-inspection response
Stay responsive, cooperative, and transparent throughout the entire process. Defensive behavior, delay in documentation, or inconsistent answers can erode trust, even in otherwise compliant systems.
Post-Inspection Response
The FDA inspection process does not end when the investigator leaves the site. What happens in the days and weeks that follow, particularly how the company responds to observations, can have a lasting impact on regulatory confidence, market access, and future inspection frequency.
Inspection Close-Out and Immediate Internal Actions
At the end of the inspection, the FDA will conduct a close-out meeting where they:
- Summarize their findings
- Present any Form FDA 483, if applicable
- Invite comments or clarifications from the company
Following this meeting, the site should:
- Hold an internal debriefing with inspection team members and management
- Document inspector remarks and verbal observations, even if not included in a 483
- Begin root cause analysis for any deficiencies discussed
- Secure and preserve all inspection-related records, logs, and communications
Preparing the FDA 483 Response
If the site receives a Form 483, the FDA expects a written response within 15 business days. A strong response should be:
- Structured by observation, addressing each point separately
- Supported by a thorough root cause analysis (RCA) using tools such as 5 Whys or Fishbone diagrams
- Include specific corrective and preventive actions (CAPAs) with clear timelines
- Provide supporting evidence, such as updated SOPs, training records, deviation reports, or validation documents
- Demonstrate management involvement and system-wide remediation, not just isolated fixes
Failure to respond adequately or on time may escalate to a Warning Letter, import alert, or product hold.
CAPA Implementation and Effectiveness Checks
Every action proposed in the response must be:
- Implemented within the committed timeline
- Documented with objective evidence
- Reviewed for effectiveness, using internal audits, trend analysis, or performance metrics
The FDA often returns to sites with prior findings for follow-up inspections. Inadequate or unsustained CAPAs are viewed as serious compliance gaps and frequently result in enforcement actions.
FDA Inspection Readiness FAQs
Can FDA Inspectors Take Photographs During an Inspection?
Yes. FDA investigators are authorized to take photographs during inspections, particularly of conditions they believe may indicate non-compliance.
Is Refusal to Allow an FDA Inspection a Violation?
Yes. Refusing or delaying an FDA inspection may result in regulatory action, including the issuance of import alerts or the withholding of application approvals.
Do Contract Manufacturers Undergo FDA Inspections?
Yes. Contract manufacturing sites listed in drug applications are subject to FDA inspections and must demonstrate independent GMP compliance.
How Long Does the FDA Typically Spend at a Facility?
Inspections typically last between 3 and 5 days, but this may vary based on site complexity, scope of inspection, and initial findings.
Can FDA Inspections Include Review of Data From Third-Party Vendors (e.g., Cloud-Based Platforms)?
Yes. If GMP data is hosted externally (e.g., in a validated cloud system), the FDA expects the regulated company to demonstrate full access, audit trails, and controls.
Conclusion
In today’s regulatory landscape, FDA inspection readiness is no longer optional—it is foundational to market access and long-term business continuity. With the FDA intensifying unannounced inspections globally, companies can no longer afford to rely on reactive compliance strategies or last-minute preparations.
Inspection readiness must be sustained as a continuous state, driven by a culture of quality, supported by reliable systems, and demonstrated through transparent, well-documented operations.
Every inspection—whether pre-approval, routine, or for-cause—is an opportunity to validate the strength of your quality system. Sites that operate with discipline, clarity, and cross-functional ownership are best positioned to meet FDA expectations confidently and without disruption.















