Good Manufacturing Practice (GMP) and Good Laboratory Practice (GLP) are two critical regulatory frameworks for ensuring quality and safety of the products. While both GMP and GLP share the common goal of safeguarding consumers and promoting best practices, they apply to different stages of the product lifecycle and encompass distinct aspects of manufacturing and testing processes.
In this article, we will explore the key differences between GMP and GLP regulations, their significance and impact on the indistry.
GMP vs GLP: Overview
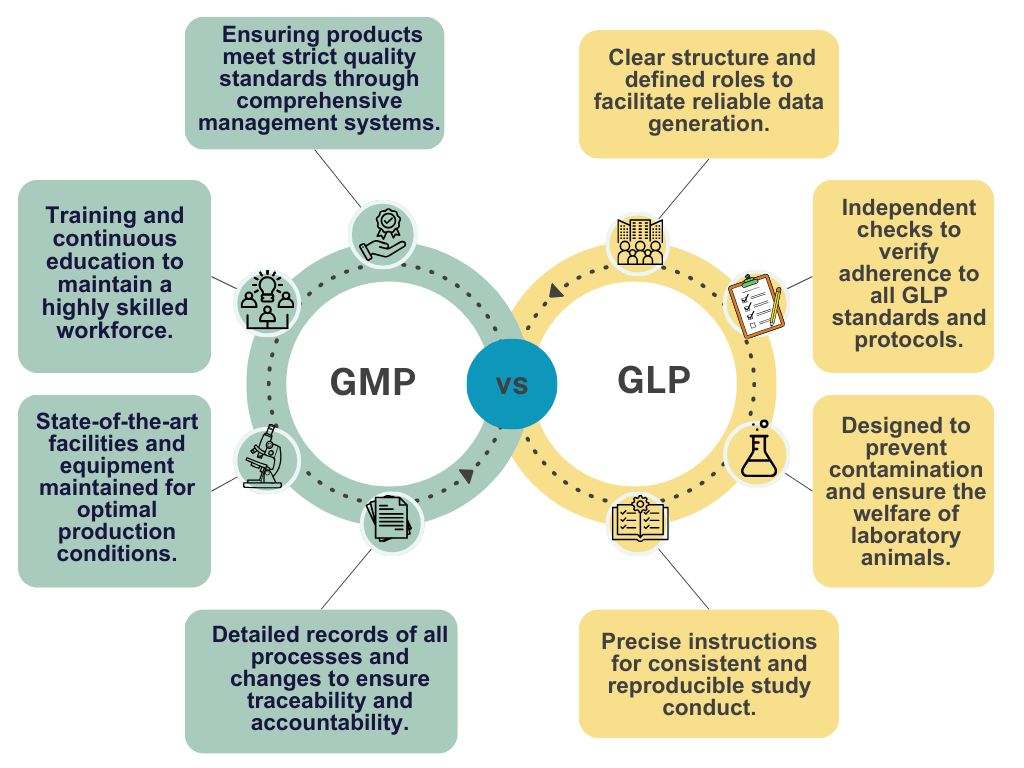
Good Manufacturing Practice (GMP) is a set of guidelines that ensure products are manufactured consistently, with quality in mind. GMP regulations cover processes and procedures, personnel training and qualifications, clear communication between departments, and more.
It aims to minimize or eliminate instances of contamination, mix-ups, and errors in the manufacturing process. GMP is crucial in industries such as pharmaceuticals, food, and cosmetics, where product safety and quality are of utmost importance.
On the other hand, Good Laboratory Practice (GLP) focuses on ensuring the reliability of laboratory tests. GLP regulations encompass documentation, record-keeping, personnel qualifications and training, equipment maintenance, and calibration records. It requires laboratories to apply their protocols uniformly across all experiments conducted. GLP plays a critical role in industries like pharmaceuticals, where non-clinical safety studies are conducted to assess the safety of drugs and chemicals.
Understanding Good Manufacturing Practices (GMP)
The primary goal of GMP is to ensure that products are consistently produced and controlled according to quality standards. It covers all aspects of the production process, from the raw materials, premises, and equipment to the training and personal hygiene of staff.
Detailed, written procedures are essential for each process that could affect the quality of the finished product. There must be systems to provide documented proof that correct procedures are consistently followed at each step in the manufacturing process – every time a product is made.
On the other hand, cGMP (current Good Manufacturing Practice) guidelines emphasize the most up-to-date quality standards.
Key Objectives of GMP
The key objectives of GMP are to minimize the risks involved in any pharmaceutical production that cannot be eliminated through testing the final product. These risks are primarily related to unexpected contamination, incorrect labels on containers, insufficient or too many active ingredients, and the possibility of the product being ineffective or hazardous to one’s health.
GMP regulations require a quality approach to manufacturing, enabling companies to minimize or eliminate instances of contamination, mix-ups, and errors. This, in turn, protects the consumer from purchasing a product that is not effective or even dangerous.
Overview of the Main Requirements of GMP
- Quality Management: The cornerstone of GMP is a robust quality management system (QMS). It ensures that products are consistently produced and controlled to the quality standards appropriate to their intended use and as required by the marketing authorization or product specification.
- Personnel Qualifications: All personnel involved in the manufacturing process must have the necessary qualifications, training, and ongoing education to perform their job competently and understand the importance of their role in ensuring product quality.
- Premises and Equipment: Manufacturing plants must be designed and maintained to suit the operations to be carried out. Their layout and design must aim to minimize the risk of errors and permit effective cleaning and maintenance to avoid cross-contamination, build-up of dust or dirt, and, in general, any adverse effect on the quality of products.
- Documentation: Detailed, written procedures are essential for each critical operation in the manufacturing process, from the receipt of materials through processing, packaging, and distribution. This documentation helps ensure traceability and accountability at all stages of production.
- Production: Production operations must be clearly defined and controlled. All critical processes are validated to ensure consistency and compliance with specifications.
- Quality Control: An independent quality control (QC) department is essential to test and verify that the raw materials, packaging materials, and intermediate, bulk, and finished products meet established quality standards.
Examples of GMP Application
- Drug Manufacturing: A pharmaceutical company producing a new medication must adhere to GMP guidelines throughout the manufacturing process. This includes controlling the quality of raw materials, ensuring the cleanliness of the production facility, validating manufacturing processes, and testing products at various stages to meet specified quality standards before the product is released to the market.
- Medical Device Production: The manufacture of a medical device, such as a heart valve, must comply with GMP to ensure the product’s safety and functionality. This involves not only the controlled environment in which the device is manufactured but also the rigorous testing of each batch and detailed record-keeping to trace each product’s manufacturing history.
Understanding Good Laboratory Practices (GLP)
Good Laboratory Practices (GLP) is a set of principles intended to ensure the quality and integrity of non-clinical laboratory studies, which are used to support research or marketing permits for products regulated by government agencies.
These practices apply to environmental studies, pharmaceuticals, cosmetics, veterinary drugs, and some medical devices. GLP provides a framework within which laboratory studies are planned, performed, monitored, recorded, reported, and archived. These practices ensure that the data and results are consistent, reliable, and reproducible and can be confidently used by regulatory authorities to assess the safety of the products.
Key Objectives of GLP
The primary objective of GLP is to promote the quality and validity of test data. It aims to ensure that studies are conducted according to universally accepted scientific principles and within the confines of a controlled environment.
This controlled environment guarantees the generation of high-quality and reliable test data to assess the safety or efficacy of the test substance on humans, animals, and the environment. GLP also aims to facilitate the mutual acceptance of test data across borders, thereby saving resources and minimizing the need for duplicating studies.
Overview of the Main Requirements of GLP
- Organization and Personnel: Laboratories must have a clear organizational structure, with well-defined roles and responsibilities for all staff members. Personnel must be adequately qualified and receive ongoing training to perform their duties competently.
- Facilities: The design and construction of the facilities must support the proper conduct of studies, including the prevention of cross-contamination and ensuring the welfare of laboratory animals.
- Equipment, Materials, and Reagents: All laboratory equipment and materials must be regularly inspected, cleaned, maintained, and calibrated to ensure their reliability and integrity in the generation of test data.
- Test Systems: Proper management of the test systems, including the organisms or cells used in studies, is essential. This includes ensuring the correct handling, housing, and care of laboratory animals.
- Test and Reference Items: The handling, storage, and use of test and reference substances must be clearly defined and controlled to ensure their stability and integrity throughout the study.
- Standard Operating Procedures (SOPs): Detailed SOPs must be in place for all critical processes and activities to ensure consistency and compliance with the predefined study protocol.
- Performance of the Study: The conduct of the study must strictly adhere to the approved study plan, documenting all deviations from the plan, with a clear justification and assessment of their impact on the study’s quality and integrity.
- Reporting of Results: The final report must accurately reflect the data collected, providing a complete, clear, and truthful account of the study conducted. It should include a discussion of the results and their relevance to the test substance’s safety or efficacy.
Examples of GLP Application
- Toxicology Studies: Before a new pharmaceutical compound can be tested in humans, it undergoes toxicological testing under GLP conditions. These studies assess the compound’s safety profile, including its potential to cause toxicity in organs and reproductive systems or through genetic damage.
- Environmental Testing: GLP is applied in studies assessing the environmental impact of new chemicals or agricultural products. For example, testing how a pesticide degrades in soil or water or its effects on non-target species would be conducted under GLP to ensure the data’s reliability for regulatory review.
GMP vs GLP: Key Aspects
While GMP and GLP share the goal of maintaining product quality and safety, there are several key differences between the two practices:
Purpose
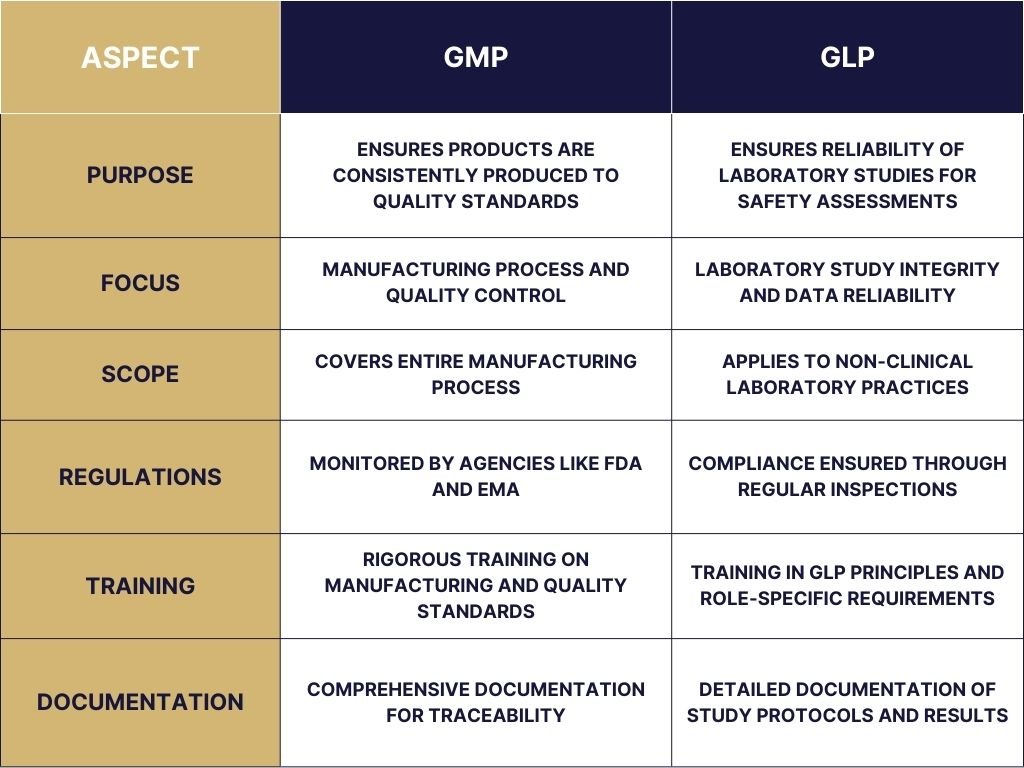
GMP: Primarily concerned with the manufacturing, quality control, and quality assurance of products. Its focus is on ensuring that products are consistently produced and controlled according to quality standards.
GLP: Specifically designed to ensure the reliability and integrity of laboratory studies. It concentrates on the laboratory setting, covering non-clinical environmental health and safety studies, as well as preclinical trials on pharmaceuticals. GLP ensures high-quality and reliable test data related to the safety of products for human health and the environment.
Objectives and Outcomes (Product Quality and Safety vs. Reliability of Laboratory Results)
GMP Objectives and Outcomes: The process-oriented nature of GMP emphasizes consistency in manufacturing processes. It’s aimed at ensuring product quality, safety, and efficacy through the control of manufacturing processes. The outcome is the consistent production of safe and effective products that comply with specified quality standards.
GLP Objectives and Outcomes: GLP is data-oriented, prioritizing the reproducibility of results across non-clinical studies. It’s aimed at ensuring the reliability, consistency, and reproducibility of data generated in laboratory studies. The outcome is credible scientific data that regulatory authorities can use to assess the safety of a product before it is marketed.
Scope
GMP regulations: Cover the entire product manufacturing process, including raw material acquisition and handling, production, quality control, and packaging. It also includes requirements for personnel qualifications, facility requirements, process controls, and quality assurance.
GLP regulations: Specifically apply to laboratory practices and the reporting of non-clinical studies. It covers aspects such as laboratory facility requirements, laboratory practices, and the documentation and reporting of experiments and studies.
Regulatory Aspects and Compliance Requirements
GMP Regulatory Aspects: Governed by stringent regulations that vary by country but are harmonized through international guidelines such as those provided by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Compliance with GMP is monitored by regulatory agencies like the FDA (U.S. Food and Drug Administration) and EMA (European Medicines Agency) through inspections and audits.
GLP Regulatory Aspects: Also regulated by national and international guidelines, with the OECD (Organisation for Economic Co-operation and Development) providing a widely accepted set of principles. Compliance is ensured through regular inspections and audits by regulatory bodies, focusing on the quality and integrity of the non-clinical laboratory data submitted for product approvals.
The OMCL Network directly enhances GLP by standardizing analytical methods and ensuring rigorous quality control across pharmaceutical testing. Its collaborative framework fosters consistency and accuracy in laboratory practices, vital for regulatory approvals and market surveillance. Through its work, the OMCL Network upholds the principles of GLP, contributing to the integrity and trustworthiness of medicinal product evaluations.
Personnel Qualifications
GMP: Employees working in manufacturing must undergo rigorous GMP training to understand and comply with quality standards. This training covers everything from personal hygiene to complex manufacturing processes.
GLP: Staff involved in GLP studies, including researchers and technicians, must be appropriately trained in GLP principles and the specific requirements of their roles to ensure the integrity and accuracy of study data.
Documentation and Record-Keeping Practices
GMP Documentation Practices: Requires comprehensive documentation for every aspect of the manufacturing process, including validation records, batch records, and quality control reports. Documentation serves as a detailed record of compliance with GMP standards and is essential for traceability and accountability.
GLP Documentation Practices: Emphasizes detailed documentation of all aspects of the study protocol, including the design, conduct, and results of the studies. Documentation under GLP includes standard operating procedures (SOPs), raw data, final reports, and audit trails. This meticulous record-keeping is crucial for the evaluation of the study’s integrity and for supporting the conclusions drawn from the data.
RELATED: Good Documentation Practices
Quality Control
GMP: Focuses on quality control of the final product, ensuring that each product batch meets the established standards for safety, quality, and efficacy.
GLP: Concentrates on the quality of data and its documentation throughout the study process, ensuring that the study design, conduct, and reported results are reliable and reproducible.
Facilities and Equipment
GMP: Specifies requirements for manufacturing facilities, including the design, maintenance, and cleanliness to prevent contamination and errors.
GLP: Establishes standards for laboratory facilities, ensuring they are suitable for conducting studies safely and effectively, with the necessary controls to prevent cross-contamination and ensure data integrity.
Audits and Inspections
GMP: Manufacturing facilities undergo regular audits and inspections to assess compliance with GMP standards, focusing on processes, personnel, and quality control measures.
GLP: Laboratories are subject to regular inspections and compliance evaluations to ensure that studies are conducted according to GLP principles, with a focus on study conduct, data integrity, and reporting accuracy.
Product Release
GMP: Finished products undergo a final quality check before being released for distribution, ensuring they are safe, effective, and of high quality.
GLP: Study data, once validated and finalized, is released for regulatory submission to support product approvals, with a focus on demonstrating safety and compliance with regulatory requirements.
Benefits of Good Manufacturing Practice and Good Laboratory Practice
Both GMP and GLP play crucial roles in maintaining product quality, safety, and compliance with regulatory standards. Here are some of the benefits of implementing GMP and GLP practices:
- Quality Assurance: GMP and GLP practices are essential components of any quality assurance program. GMP ensures that products are manufactured consistently and safely, strictly adhering to quality standards. GLP ensures the reliability and integrity of laboratory studies, ensuring that data generated from experiments is accurate and reproducible.
- Compliance with Regulatory Standards: Adhering to GMP and GLP standards ensures compliance with regulatory requirements. This compliance is essential for industries such as pharmaceuticals, food, and cosmetics, where regulatory authorities set stringent standards for product safety and quality.
- Consumer Safety and Confidence: Implementing GMP and GLP practices instill trust and confidence in consumers. By following these practices, companies demonstrate their commitment to producing safe and high-quality products. This, in turn, enhances consumer safety and satisfaction.
- Cost Savings: Implementing GMP and GLP practices can lead to cost savings. By ensuring that manufacturing processes are efficient and error-free, companies can minimize waste, avoid product recalls, and reduce the costs associated with non-compliance.
- Improved Efficiency: GMP and GLP practices promote efficient manufacturing and laboratory operations. Companies can streamline processes, reduce errors, and improve overall efficiency by following standardized procedures.
Implications of not Following GMP and GLP
Failure to follow GMP and GLP practices can have serious consequences for companies, including:
- Regulatory Non-Compliance: Non-compliance with GMP and GLP regulations can result in regulatory action, including warning letters, fines, and penalties. Regulatory authorities may conduct inspections and audits to assess compliance with these standards.
- Product Quality Issues: Not following GMP and GLP practices can lead to product quality issues, such as contamination, mix-ups, and errors in the manufacturing process. These issues can compromise the safety and efficacy of products, leading to potential harm to consumers.
- Reputational Damage: Companies that do not adhere to GMP and GLP standards may suffer reputational damage. Negative publicity, product recalls, and safety concerns can erode consumer trust and confidence in the company and its products.
- Legal Consequences: Failure to comply with GMP and GLP regulations can result in legal consequences, including lawsuits and liabilities. Companies may face legal action from consumers, regulatory authorities, or other stakeholders if their products are found to be unsafe or of poor quality.
FAQ: GMP vs GLP
Can a Facility Be Both GMP and GLP Compliant?
Yes, a facility can be both GMP and GLP compliant if it conducts both manufacturing processes and non-clinical laboratory studies. However, the facility must meet the distinct requirements of each set of standards, which involves having separate quality management systems, documentation, and controls appropriate for manufacturing and laboratory operations.
What Are the Challenges of Adhering to GMP and GLP?
Challenges include navigating the complex regulatory landscape, managing the high costs of compliance, ensuring the adequacy of quality control measures, and keeping up with changes in regulations. Global companies must also manage these standards across different countries and regulatory environments.
How Often Are GMP and GLP Facilities Inspected?
The frequency of inspections for GMP and GLP facilities can vary based on the regulatory authority, the country, and the specific activities of the facility. Typically, regulatory agencies conduct inspections every two to three years, but this can be more frequent if there are previous instances of non-compliance or if the facility is involved in high-risk manufacturing or testing activities.
What Does GMP Mean in Labs?
In laboratories, Good Manufacturing Practice (GMP) refers to the strict guidelines that govern the manufacturing processes, quality control, and quality assurance of pharmaceuticals and related products. It ensures that products are consistently produced and controlled to quality standards. GMP in labs mainly involves quality control testing, production of clinical trial materials, manufacturing of active pharmaceutical ingredients (APIs), and any process that directly affects the safety and efficacy of the final product. GMP emphasizes documentation, personnel training, equipment maintenance, and facility cleanliness to prevent contamination and ensure product integrity.
Related: QA vs QC
GMP vs GLP: Final Thoughts
GMP and GLP are two sets of standards that play crucial roles in ensuring product quality, safety, and compliance with regulatory requirements. While GMP focuses on manufacturing processes, quality control, and quality assurance, GLP focuses on laboratory practices and the reliability of non-clinical studies.
Both GMP and GLP are essential for maintaining consumer safety, product integrity, and regulatory compliance in industries such as pharmaceuticals, food, and cosmetics. By implementing GMP and GLP practices, companies can ensure that their products meet the highest standards of quality and safety, instilling trust and confidence in consumers. Compliance with GMP and GLP regulations is not only a legal requirement but also a strategic imperative for companies operating in highly regulated industries.















