The pharmaceutical industry demands highly accurate and reproducible analytical techniques to ensure product quality and patient safety. Chromatography plays a pivotal role in meeting these expectations, but inconsistent practices can lead to compliance issues, product recalls, and market disruptions.
Good Chromatography Practices help align laboratory workflows with regulatory frameworks, ensuring robust data integrity and operational efficiency. In addition, with increasing global regulatory scrutiny and market demands, implementing Good Chromatography Practices can significantly reduce the risks of batch failures, ensuring the timely release of products into the market.
Benefits of Good Chromatography Practices in the Pharma Industry
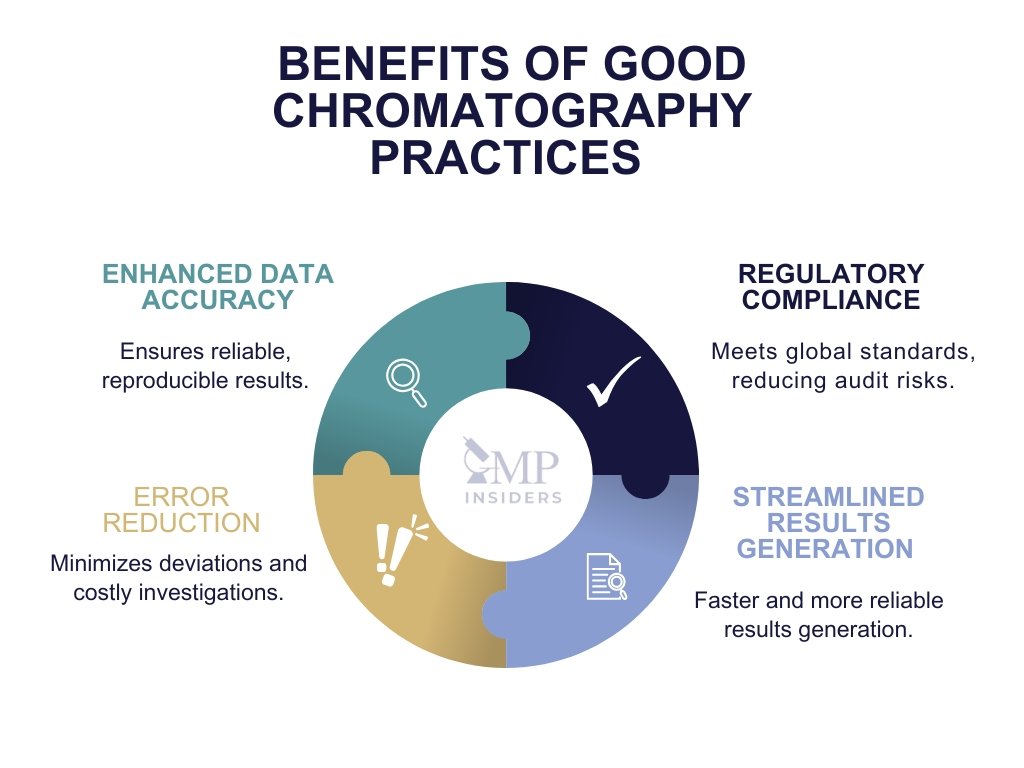
Good Chromatography Practices offer significant benefits, including improved data accuracy, minimized errors, and enhanced compliance with regulatory standards. By adhering to Good Chromatography Practices, laboratories can effectively minimize operational risks, maintain high standards of data integrity, and expedite the release of quality products.
Reduction of Errors, Non-Compliance Risks, and Costs
Implementing standardized chromatographic procedures minimizes errors during data acquisition and processing, thereby reducing the risk of deviations and costly investigations. It ensures seamless audits, limiting the risk of non-compliance citations that could lead to financial penalties or operational downtime.
Data Integrity
High-quality chromatographic data supports robust data integrity through accurate data acquisition, minimizing errors, and ensuring traceability. Good Chromatography Practices enhance data reliability, providing consistent and reproducible results, which is crucial for maintaining regulatory compliance.
In recent years, data integrity issues have been a major focus of regulatory authorities, with the FDA issuing multiple warning letters annually related to irregularities in data management, traceability gaps, and improper record handling. Ensuring compliance with Good Chromatography Practices helps organizations avoid such violations, safeguarding the quality of analytical results and protecting public health.
Streamlined Results Generation
Efficient Quality Control (QC) processes in chromatography reduce operational bottlenecks, enabling faster and more reliable result generation. Standardization in chromatographic methods, equipment handling, and sample management supports repeatability and minimizes discrepancies. When automated chromatographic processes are integrated with Good Chromatography Practices, they streamline documentation, result reporting, and system suitability testing (SST), leading to more agile and compliant QC operations, ultimately enhancing productivity and consistency across manufacturing.
Compliance with Regulatory Requirements
Following Good Chromatography Practices ensures compliance with regulatory requirements, particularly the guidelines set by the World Health Organization (WHO). Regulatory bodies increasingly focus on data integrity, audit trails, and chromatographic validation. Good Chromatography Practices help laboratories meet these stringent requirements and ensure all chromatographic methods are fully validated, documented, and audit-ready.
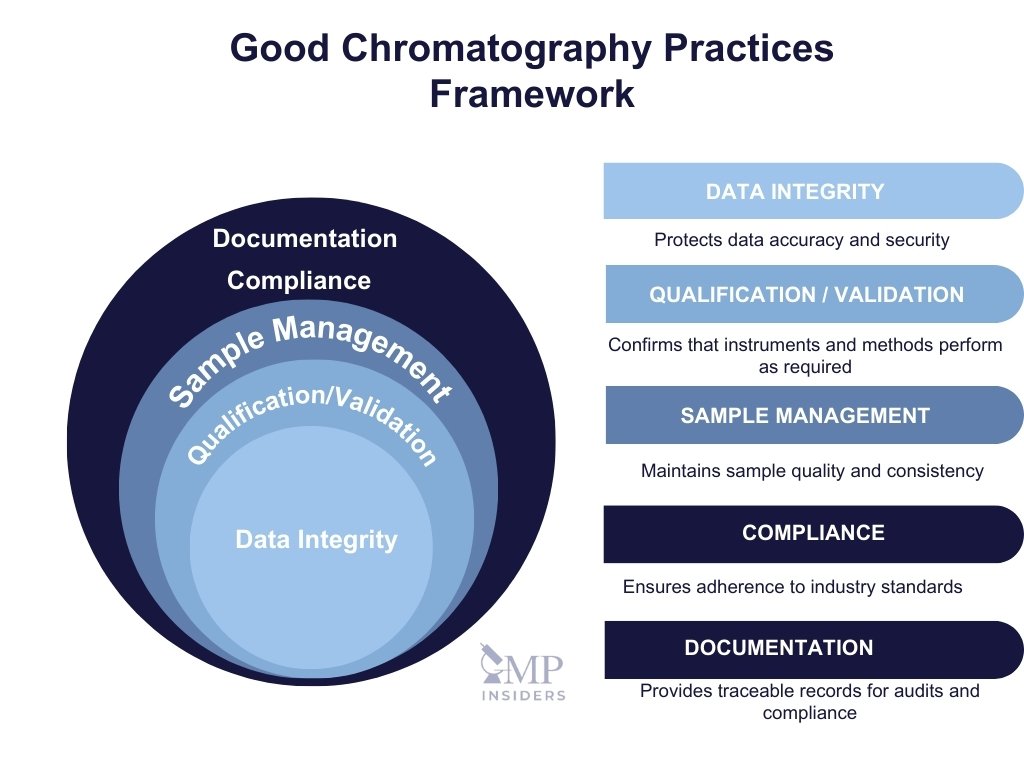
Qualification, Validation, Maintenance, and Calibration
Chromatographic instruments must consistently perform within their intended specifications to ensure accurate and reliable analytical results. Achieving this requires a structured approach involving qualification, validation, regular maintenance, and calibration. Each activity plays a critical role in building trust in the data generated and ensuring regulatory compliance.
Supplier Assessment and Vendor Qualification
Vendor qualification begins with a User Requirement Specification (URS) provided by the user, outlining performance, maintenance, and calibration needs. The Installation Qualification (IQ) is jointly performed by the vendor and user to ensure the equipment meets predetermined specifications. Supplier assessment is also crucial for all consumables used in chromatography, ensuring they meet quality standards. A clear User-Vendor Responsibilities Agreement (UVRA) helps define the roles in maintaining and servicing equipment, preventing misunderstandings during audits.
RELATED: Supplier Qualification in GMP
External and Internal Qualifications (Periodic Checks)
Laboratories must implement a dual-layer qualification strategy—routine internal qualifications following Standard Operating Procedures (SOPs) and periodic assessments from certified external providers. Depending on the frequency of use for the instruments, end users need a suitable strategy (internal qualification) to detect any performance changes between requalifications performed by external service providers.
A risk-based approach helps determine the frequency and depth of qualification activities, ensuring critical GMP instruments are assessed more frequently while optimizing resources for non-critical instruments. This approach ensures instruments are qualified according to industry standards without disrupting lab operations.
Preventive Maintenance
Preventive maintenance is a proactive approach to maintaining equipment performance, reducing the risk of unexpected breakdowns, and extending the lifespan of chromatography instruments.
Bundled Maintenance with Qualification: Aligning preventive maintenance with periodic qualification checks reduces downtime and improves efficiency. Maintenance records should document each action, including parts replaced and observations, providing a complete maintenance history that aids in long-term performance tracking.
Component Replacements: Monitoring and replacing essential parts such as pistons, seals, filters, and lamps should be done based on usage or manufacturer recommendations. Such information is available and monitored in most Chromatography Data Systems (CDS), where end users can track parameters like lamp hours, pumped volume, and number of injections. This practice prevents common issues like leaks, pressure fluctuations, and baseline noise that can impact analytical accuracy.
Traceability in Instrument Calibration
All calibrations should be traceable to international standards, such as ISO 17025, ensuring consistent performance across instruments and facilities. This traceability is crucial during regulatory inspections to demonstrate compliance with global benchmarks.
Alarm Systems and Notifications
Automated systems should provide alerts for calibration and maintenance deadlines, preventing equipment from going out of qualification. These alerts help labs stay proactive, ensuring smooth operations without regulatory interruptions.
Documentation in Chromatography
Proper documentation in chromatography ensures full traceability of data and processes, aligning with regulatory requirements. Each chromatographic analysis—whether related to production, batch testing, or R&D—requires comprehensive records detailing both the acquisition and processing stages. This guarantees that any result can be traced back to the original conditions and users involved. Down below are some of the necessary documentation elements:
- Instrument ID: Each instrument must be uniquely identified in the documentation to trace the source of data or errors back to the correct equipment.
- Product Name & Batch Number: Linking chromatographic runs to a specific product and batch ensures traceability in case of deviations or recalls. Regulatory bodies require every test to be associated with the batch it supports.
- User Acquiring the Chromatogram: This user sets up and initiates the chromatographic sequence. Documentation must reflect accountability for the setup conditions.
- User Processing the Chromatogram: A chromatogram often undergoes post-acquisition processing (such as peak integration and baselining). Documentation ensures transparency about who was responsible for any manual integrations.
- User Printing or Approving the Chromatogram: If hard copies are required for audits or internal review, the responsible user must be documented for traceability.
These records support audit trails, accountability, and reliability, critical in regulated industries such as pharmaceuticals.
Reagents Documentation
All reagents (solvents, buffers, standards) used in the chromatographic process must be documented to ensure quality and consistency. This includes:
- Lot numbers and expiry dates to ensure traceability and avoid expired or non-compliant reagents.
- Preparation records (e.g., dilutions or mobile phase preparation).
- Storage conditions to verify that reagents are kept under suitable conditions (e.g., temperature-sensitive solvents).
SEE ALSO: Setting Expiry Dates for QC Reagents
Chromatographic Column Documentation
Chromatographic columns are a key element in the process, and poor column management can affect results. Documentation should include:
- Column brand, type, and lot number: To ensure reproducibility between runs.
- Usage history: Columns degrade over time, so records of column usage (including the number of injections) help track performance.
- Cleaning and storage conditions: Certain columns require specific storage conditions, which must be recorded to maintain column integrity.
CDS Column Libraries
Modern Chromatography Data Systems (CDS) offer column libraries where all column-related information is centrally stored and linked to specific chromatographic sequences. This reduces the risk of linking incorrect columns to generated data and saves time during setup.
Instrument Logbooks
Instrument logbooks are essential tools for tracking the usage, maintenance, and performance of chromatography instruments. They ensure that each activity related to an instrument is well-documented, allowing traceability of both equipment performance and the products tested.
Key Elements to Include in Instrument Logbooks
- Instrument Identification:
- Unique instrument ID, serial number, and model information for tracking the exact equipment used.
- Usage History with Tested Products and Batches:
- Product Name and Batch Number: Each entry should document the product and specific batch being tested to connect the chromatographic data with the corresponding batch record. This ensures traceability, which is crucial during investigations, especially for out-of-specification (OOS) results or product recalls.
- Test Purpose: Mention whether the test was for raw material qualification, in-process testing, finished product release, or stability studies.
- Analysis Date and Time:
- The date and time of each use should be recorded to ensure all testing is accounted for chronologically and to prevent data gaps.
- Analyst/User Information:
- The name (or user ID) of the analyst responsible for operating the instrument during each session, establishing accountability.
- Calibration, Qualification, and Performance Checks:
- Records of calibration (including any standards or reference materials used) and periodic qualifications such as IQ, OQ, and PQ.
- Results of daily performance checks (e.g., system suitability tests) to confirm that the instrument was functioning properly at the time of analysis.
- Maintenance and Repairs:
- Detailed records of preventive maintenance, repairs, and part replacements.
- Information on any downtime and the actions taken to address it.
- Deviations and Issues:
- Documentation of any deviations, malfunctions, or unexpected issues encountered during the use of the instrument, along with corrective and preventive actions (CAPA).
- Cleaning Records:
- For instruments that require cleaning between different products or batches, records of cleaning activities (including solvents used) should be documented to prevent cross-contamination.
Electronic Records
Electronic records are essential for maintaining data integrity and traceability throughout the chromatographic workflow. They capture everything from instrument settings and raw data to audit trails and result reports.
In chromatography, electronic records can be broadly categorized into:
- Static Records: Immutable records that serve as fixed, unalterable snapshots of data. These include PDF reports, batch summaries, audit trail exports, etc. They are vital for audits and official documentation.
- Dynamic Records: Records that are subject to re-analysis, adjustment, or further processing, such as chromatograms stored in a CDS. Dynamic records allow analysts to adjust integration parameters, but must be managed carefully with robust audit trails to track all changes.
Data Integrity
Data integrity refers to the completeness, accuracy, and consistency of data throughout its lifecycle. In chromatography, maintaining data integrity ensures that results are reliable and traceable, safeguarding product quality and regulatory compliance. Adopting best practices, such as following ALCOA++ principles, implementing robust user management, and maintaining audit trails, ensures that data is both trustworthy and defensible during audits.
ALCOA++ Principles
The ALCOA++ framework outlines key principles for maintaining data integrity, helping laboratories meet regulatory requirements. These principles ensure that data is not just accurate but also appropriately documented and accessible over time.
Attributable: Every entry or change to data must be linked to a specific user, showing who performed an action.
Legible: Data must be clear and understandable throughout its lifecycle. This applies to both electronic and hard copy records.
Contemporaneous: Data must be recorded in real time, as activities happen, to avoid gaps or inaccuracies.
Original: Original data (raw data) must be preserved as evidence of the first measurement or action.
Accurate: Data must reflect the true values or activities without manipulation or omissions.
The “++” extension includes additional principles:
Complete: All relevant data, including failed attempts, deviations, and reprocessing efforts, must be recorded.
Consistent: Data must follow the same format and structure across systems and processes.
Enduring: Data must be preserved in readable form throughout the required retention period.
Available: Data must be accessible for audits or inspections when needed.
Traceability: Data is traceable throughout the process and life cycle, including changes.
Administrative Tasks
Effective administrative controls are essential for ensuring data integrity, especially in laboratories with limited personnel. Assigning appropriate user roles and managing access privileges ensures that only authorized personnel can make changes to critical data.
Who Can Be an Administrator?
Segregation of Duties: Ideally, the system administrator role should be assigned to someone outside the operational or analytical teams to avoid conflicts of interest. However, in small laboratories with limited personnel, the role may be taken by a qualified analyst or supervisor.
Competency Requirements: Administrators must be trained in both IT and data integrity principles to ensure that they properly manage access, passwords, and audit trails.
Documented Procedures: All administrative activities (e.g., adding new users, modifying privileges) must be documented to ensure traceability. These procedures should align with the laboratory’s Standard Operating Procedures (SOPs).
Implementing Administrative Tasks in Laboratories with Limited Personnel
In smaller labs, individuals may need to hold multiple roles. Ensure that every administrative action is recorded with justifications and subjected to periodic review by a second person (e.g., a supervisor).
Periodic Audits: Conduct internal audits of administrative tasks to identify potential risks, such as unauthorized access or improper privilege assignments.
External Support: Use external service providers for system administration or backup management if in-house expertise is limited.
User Access and Privileges
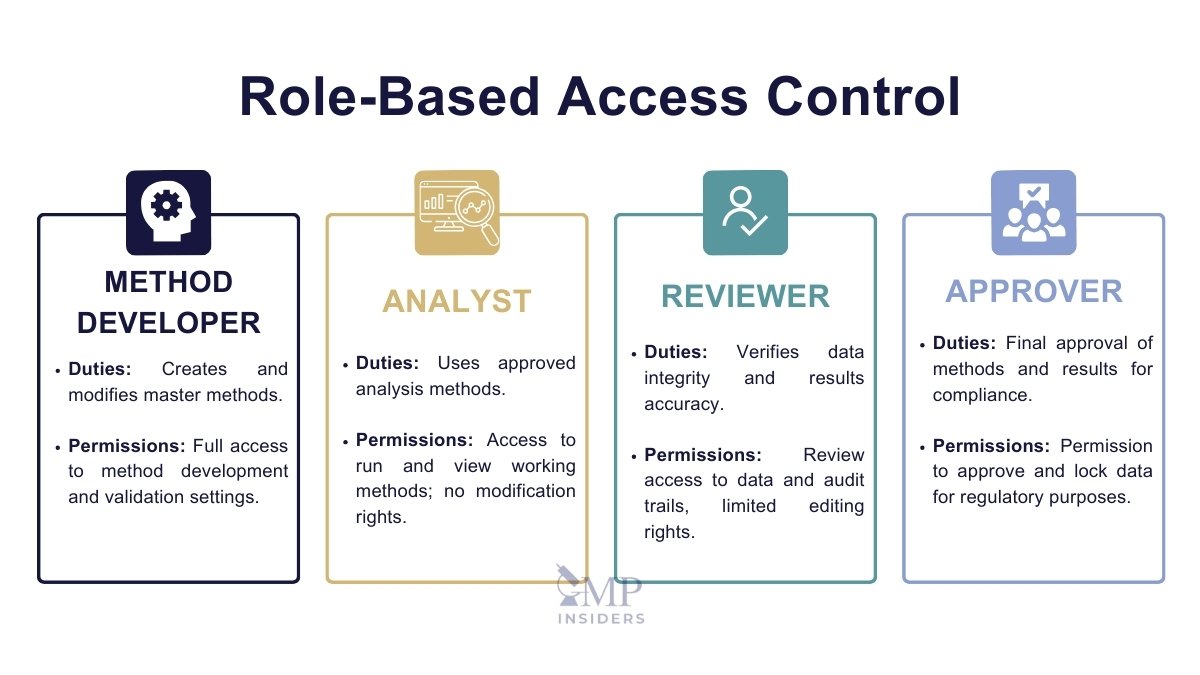
Managing user access is critical to prevent unauthorized changes to sensitive data or processes. Each user should have access only to the systems and functions necessary to perform their job (the principle of least privilege).
Role-Based Access Control: Assign roles (e.g., analyst, reviewer, approver) to ensure clear separation of duties.
User Privilege Management: Regularly review user access and privileges to ensure inactive users are removed and privileges are consistent with the user’s responsibilities. All changes to user access or privileges must be documented through a formal change control process.
Temporary Privileges: Implement time-limited privileges for temporary access (e.g., for contractors or external auditors).
Account Lockout Policies: Define account lockout procedures to protect against unauthorized login attempts.
Passwords
Proper password management ensures secure access to CDS. Password policies should align with industry standards for cybersecurity and GMP compliance.
Password Expiration: Set regular expiration intervals (e.g., every 60 to 90 days) to prevent unauthorized access. Users should receive reminders before expiration to update their passwords.
Password Complexity: Require passwords to be at least 8-12 characters long and include a combination of letters, numbers, and special characters to enhance security.
Prohibited Password Reuse: Implement policies that prevent reuse of recent passwords to avoid weak password rotation.
Failed Login Attempts: Lock accounts after a defined number of failed login attempts to prevent unauthorized access through brute force attacks.
Electronic Signatures
Electronic signatures (e-signatures) are a digital equivalent of handwritten signatures, providing accountability and traceability. They are essential for signing electronic records, such as chromatograms, batch reports, and validation documents.
Regulatory Requirements: E-signatures must meet 21 CFR Part 11 requirements, ensuring that they are unique, traceable, and secure.
Multiple Signings for Critical Data: Chromatograms and reports may require double signatures—one from the analyst who performed the test and another from the reviewer or approver to confirm accuracy.
Security Measures: Ensure that each e-signature is linked to an authenticated user and that audit trails capture the signature’s application time and purpose.
Sign-Off Workflows: Use CDS workflows that require multiple levels of review (e.g., analyst, reviewer, approver) to prevent unintentional errors and ensure compliance.
Audit Trails
Audit trails capture detailed logs of all actions performed within a chromatography system, ensuring traceability and accountability. They are essential for maintaining data integrity by providing a record of changes made to methods, sample sets, reports, and other critical activities.
Scientific Rationale for Changes: When changes are made to chromatographic data (e.g., manual peak integration), users must provide scientific justifications for the adjustments. This ensures transparency and prevents data manipulation.
Active Audit Trails: Enable audit trails for data, methods, batches (sample sets), and reports to capture changes, including the time, user, and reason for each change.
Regular Review: Regulatory bodies require periodic review of audit trails to detect potential issues, such as unauthorized changes or unusual activity.
Compliant CDS Systems: Ensure that the CDS platform automatically logs changes and maintains complete audit trails that cannot be altered or deleted.
Backup
Maintaining regular backups of electronic records is crucial for data preservation and compliance with 21 CFR Part 11. Backups ensure that data is available even in case of system failures, data corruption, or cyberattacks.
Backup Locations:
- Primary Backup: Stored onsite for quick access.
- Secondary Backup: Stored at an offsite location to protect against disasters at the primary site.
Regular Backup Frequency: Daily, weekly, or event-based backups ensure that recent data is preserved.
Assessment of Backup Intervals: Define backup intervals based on the volume and criticality of data. For example, batch analysis data may require more frequent backups than routine maintenance logs.
Validation and Testing: Periodically test the backup system to ensure that data can be restored successfully within a defined timeframe.
Automated Backup Systems: Use automated, validated backup systems to minimize the risk of human error and ensure that backups are performed consistently.
Chromatographic Column Management
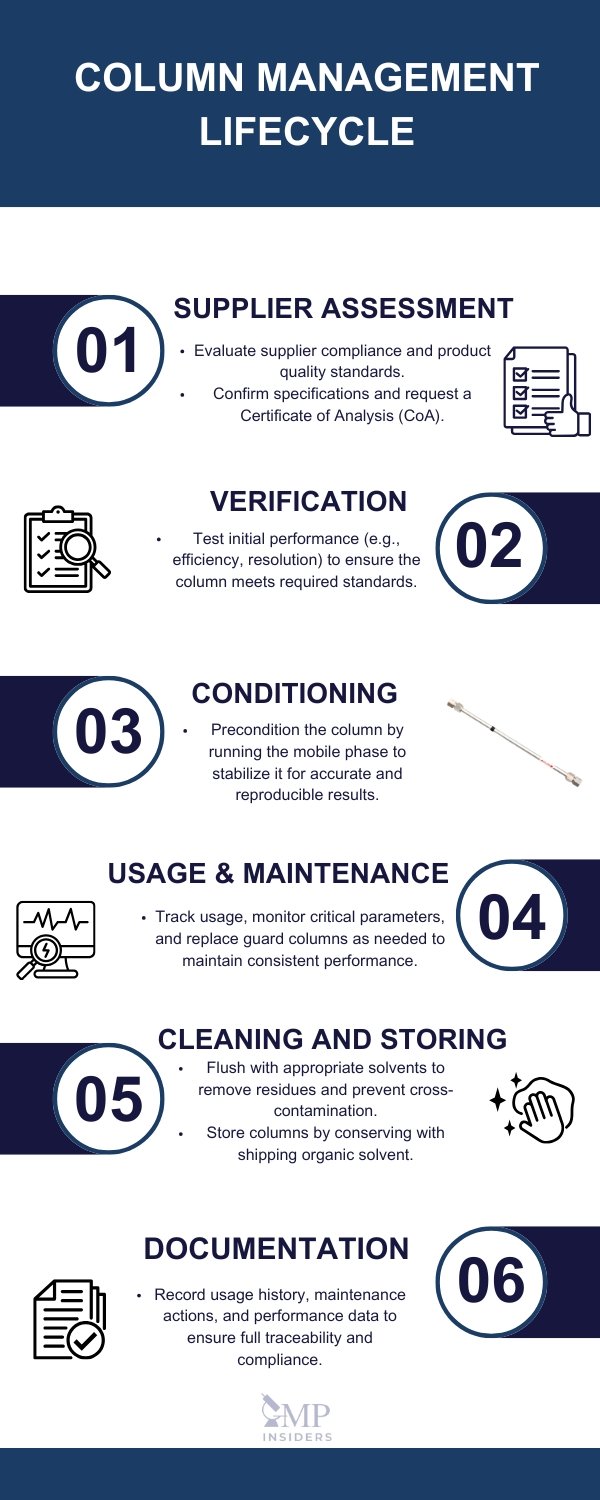
Chromatographic columns are critical components of analytical chromatography systems, directly affecting the quality and reproducibility of results. Proper management, handling, and documentation of columns help maintain column performance, extend their lifespan, and ensure regulatory compliance.
Supplier Assessment and Verification
Selecting and verifying suppliers is the first step toward ensuring that chromatography columns meet regulatory and quality expectations. Columns must be assessed before ordering and their suitability verified before use in critical applications.
Supplier Qualification: Evaluate the supplier’s reputation, manufacturing standards, and compliance with GMP.
Certificate of Analysis (CoA): Request a CoA to confirm key specifications, such as particle size, column dimensions, and performance parameters.
Verification of Suitability Pre-Use
Performance Testing: Verify key parameters (e.g., column efficiency, retention time, resolution) by running system suitability tests with reference standards.
Compatibility Check: Ensure the column is compatible with the mobile phases and analytes to be tested. Columns that are not chemically compatible can degrade quickly or produce erroneous results.
Documentation: Record all tests and verifications in column logbooks or chromatography data systems (CDS) to ensure traceability.
Use, Handling, and Storage of Columns
Proper handling, use, and storage are essential to preserve the performance of chromatographic columns and prevent damage. Careful management minimizes the risk of contamination, mechanical damage, and deterioration of column packing material.
Conditioning of Columns
Preconditioning: Before the first use, condition the column by flushing it with the intended mobile phase for a specified duration to equilibrate the stationary phase.
Gradient Equilibration: If using gradient elution, ensure the column is properly equilibrated between runs to maintain reproducibility.
Proper Storage with Closed Fittings
Short-Term Storage: Use closed end fittings or seal the column with plugs to prevent drying out or contamination when the column is not in use.
Long-Term Storage: For long-term storage, flush columns with storage solvents (e.g., methanol or acetonitrile) as recommended by the manufacturer. Ensure the columns are labeled with last use date and storage conditions.
Avoiding Mechanical Shocks
Transport and Handling: Handle columns carefully to avoid mechanical shocks. Drops or falls can damage the column bed, resulting in uneven flow paths or packing issues, leading to peak broadening or decreased resolution.
Use of Pre-columns (Guard Columns)
Prolonged Shelf Life: Install pre-columns or guard columns to capture impurities from the mobile phase or sample, protecting the main analytical column.
Replacement Schedule: Guard columns should be replaced regularly to avoid breakthrough of contaminants to the main column.
Use of Dedicated Columns per Product
Product-Specific Columns: Assign dedicated columns for specific products or analytes to avoid cross-contamination. This approach is critical for regulated industries where product purity is of great importance.
Optimal Use: Track the usage of dedicated columns to maintain consistent performance and prevent mixing of incompatible substances across runs.
Washing of Columns
Routine column washing helps maintain performance and prevent issues caused by residual buffers or contaminants. Following the correct procedures can significantly prolong the life of a column.
Buffers and Salt Residues: After using mobile phases containing buffers or salts, thoroughly flush the column with water to prevent salt precipitation. Follow with organic solvents (e.g., acetonitrile or methanol) to remove any remaining residues.
Reverse Flushing (if applicable): Some columns can tolerate reverse flushing to remove particulates or clogging material from the inlet. Refer to the manufacturer’s guidelines to confirm whether this is appropriate.
Scheduled Maintenance: Establish a regular washing schedule based on the column usage and types of samples analyzed.
Column Data Records (Logbooks)
Accurate record-keeping for columns is essential to ensure traceability, optimize performance, and identify degradation trends over time. This documentation is often stored in logbooks or CDS column libraries.
Logbook Entries: Each time a column is used, log the product, batch, date, and analyst in the column logbook. This ensures traceability and helps monitor trends over the column’s lifespan.
CDS Column Libraries: Modern CDS platforms offer column libraries where usage history, performance data, and qualification status are stored. This reduces manual logging errors and improves compliance.
Monitor Parameters Indicating Column Degradation
Monitoring key parameters can help detect early signs of column degradation and ensure timely replacement, preventing analytical errors.
Theoretical Plate Number (N): Decreasing plate numbers indicate a reduction in efficiency, possibly due to column fouling or packing deterioration.
System Pressure: Monitor backpressure trends to identify clogging or changes in flow resistance. A gradual increase in pressure suggests column blockage or wear.
System Suitability Parameters (e.g., Resolution): For parameter or product dedicated columns, track resolution between critical peaks. Deteriorating resolution may indicate that the column needs replacement or regeneration.
Monitor the Number of Injections per Column
Injection Tracking: Log the number of injections to assess when a column approaches its expected lifespan.
Performance Threshold: Establish thresholds for maximum injections based on historical data and manufacturer recommendations. Regular performance tests (e.g., with system suitability standards) should confirm if the column still meets criteria after a certain number of injections.
Reagents
Reagents (mobile phases, buffers, solvents, water, reference standards) must meet strict quality standards, as impurities or incorrect preparation can introduce variability or errors in the analysis.
Water Quality
Type of Water: The water used in chromatography should typically be HPLC-grade water to prevent contamination and ensure compatibility with columns and detectors.
Monitoring Water Quality: Regular testing of water quality for pH, conductivity, and total organic carbon (TOC) ensures consistency.
Storage and Use: Water should be stored in clean, closed containers to prevent microbial contamination. Avoid prolonged storage to maintain purity.
Preparation of Mobile Phases
Accuracy of Composition: Mobile phases must be prepared according to the method specifications, ensuring consistent ratios of solvents, buffers, or additives. Use graduated cylinders, calibrated balances, and pH meters for accuracy.
Filtration and Degassing: Filter mobile phases using 0.45 µm or 0.22 µm filters to remove particulate matter. Degassing (via vacuum or sonication) prevents air bubbles from interfering with the chromatographic flow.
Labeling and Storage: Properly label mobile phases with preparation date, composition, and expiry date. Store them according to stability guidelines, often under cool and dark conditions.
Purity of Solvents
HPLC-Grade Solvents: Use HPLC- or LC-MS-grade solvents to minimize interference from impurities. Lower-grade solvents may introduce contaminants that affect system performance or degrade columns.
Monitoring for Contaminants: Perform regular quality checks on solvents for UV absorbance, fluorescence, or residue levels to ensure they meet analytical requirements.
Handling and Disposal: Solvents should be handled with care to avoid contamination and disposed of according to environmental safety protocols.
Sample Management
Accurate sample preparation and handling are critical to ensuring reproducibility and reliable chromatography results. Proper sample management minimizes variability and ensures compliance with regulatory guidelines. Below are the key aspects of managing test samples, reference standards, system suitability solutions, placebos and blanks.
Test Solutions
Test solutions contain the actual analytes being measured and must be prepared carefully to ensure accurate quantification and reproducibility of results.
Preparation of Test Solutions:
- Prepare test solutions according to the analytical method using precise volumes and concentrations.
- Use high-quality solvents and properly calibrated equipment during preparation to avoid variability.
- Ensure test solutions are homogeneous by properly mixing them before use.
- Filter test solutions with high-quality HPLC filters (0.45 µm or 0.22 µm) with verified analyte and solvent compatibility
Test Solutions in Replicates (Evaluation of Results)
- Prepare and analyze replicate test solutions to assess the precision of the method.
- Replicates help identify variability in sample preparation or instrument performance and ensure that results are consistent.
Stability of Test Solutions
- Certain test solutions may degrade over time, leading to inaccurate results. Perform stability studies to determine how long solutions remain stable under specific conditions.
- Store solutions under controlled temperature and light conditions to maintain integrity. For sensitive samples, use amber glassware to prevent photodegradation.
Reference Standard Solutions
Reference standards are used to ensure analytical consistency and validate the performance of the chromatography system over time.
Preparation of Reference Standards
Prepare reference standards carefully, using certified materials with known purity and concentration.
Follow internal lab rules for similarity determination (standard preparation in duplicate), ensuring consistency in concentration and preparation steps between different batches of reference standards.
Dilution and Storage Conditions: Standards should be diluted accurately using calibrated pipettes and stored under conditions specified by the manufacturer or laboratory SOP.
Stability of Reference Standards
Some reference standard solutions (prepared in target concentrations) may degrade over time; therefore, stability studies are essential.
Label with expiry dates and track their usage in logbooks or CDS systems to avoid using expired standards.
SEE ALSO: Primary vs Secondary Reference Standards in GMP Labs
System Suitability Solutions (SST)
System suitability tests (SST) verify that the chromatographic system is functioning correctly before analyzing actual samples.
- SST solutions must remain chemically stable throughout their use to provide reliable performance assessments.
- Prepare SST solutions as per method-specific guidelines and store them under appropriate conditions to prevent degradation.
- Monitor the performance of SST solutions by tracking critical parameters like resolution, peak symmetry, and retention time during each run.
Blank and Placebo Solutions
Blank and placebo solutions play important roles in ensuring accuracy and specificity in chromatographic methods.
What Are Blanks and Their Role in Chromatography?
Blanks contain only the solvent or mobile phase without any analyte. They are used to establish baseline noise levels and detect contamination or carryover from previous injections.
Blank injections are crucial to demonstrate that peaks in the chromatograms are due to analytes and not interferences from solvents or equipment.
Importance of Placebo Runs in Related Substances Determination
Placebos contain all formulation components except the active pharmaceutical ingredient (API). They are used to ensure that excipients or formulation matrices do not interfere with the detection or quantification of related substances.
Placebo runs help differentiate between impurities originating from the API and those from excipients, supporting method specificity and regulatory compliance.
Chromatographic Methods
In chromatography, methods serve as the base of consistent, reproducible analysis. To ensure reliability and regulatory compliance, laboratories must adopt controlled workflows for creating, managing, and using methods. This includes assigning user access levels, developing robust master and working methods, and employing systematic method lifecycle management.
Privileges for Everyday Analysis
Analysts should have restricted access to approved working methods only, ensuring that they follow validated procedures without unintentional changes.
By implementing controlled method use with appropriate access privileges, laboratories can ensure that chromatographic methods are executed consistently, reducing variability between runs and preventing deviations.
Master Methods and Working Methods
Chromatographic methods evolve from master methods to working methods, each serving a distinct purpose to ensure traceability, consistency, and control.
Master Methods
A master method is the original, validated version of the method, containing detailed instructions on instrument settings, mobile phases, and processing parameters. This method is often developed during analytical method validation and remains locked to prevent modifications unless scientifically justified.
Working Methods
A working method is a copy of the master method, used for routine analysis. It is typically adapted to include batch-specific parameters or product-specific details, while the core conditions remain unchanged.
Controlled Copies: Each working method must be traceable back to the original master method, with any deviations or changes documented and approved.
Method Processing Parameters
Method development should also focus on data acquisition and processing parameters, which directly impact peak integration and quantification. These parameters define how data is collected and analyzed, influencing the overall quality of results.
Importance of Studying Previous Product Analyses
Review historical data to understand analyte behavior, baseline noise, retention times, and peak shapes. This helps in optimizing parameters such as peak detection thresholds, integration limits, and smoothing factors, ensuring consistent peak identification across batches.
Lifecycle of Methods
Chromatographic methods follow a lifecycle approach, evolving from development and validation through routine use and periodic revalidation. Investing in system automation and automated integration reduces manual interventions and ensures consistent results over time.
Automated Integration and Data Processing
Automating peak integration minimizes human error, providing consistent and unbiased data processing. CDS platforms with automated integration features allow for predefined parameters (e.g., baseline algorithms, peak detection thresholds) to be consistently applied across all chromatograms (per sample set).
RELATED ARTICLE: Data Integrity for Chromatographic Peak Integration
Continuous Improvement and Revalidation
Methods should be periodically reviewed and revalidated to account for changes in product formulations, regulatory requirements, or equipment. Regular analysis of system suitability results can reveal trends that indicate the need for method adjustment (e.g., chromatographic parameters alterations).
Documentation Throughout the Lifecycle
Document each phase of the method’s lifecycle, from initial development through routine use and retirement. Use controlled change management procedures to ensure that any modifications to the method are scientifically justified, approved, and documented.
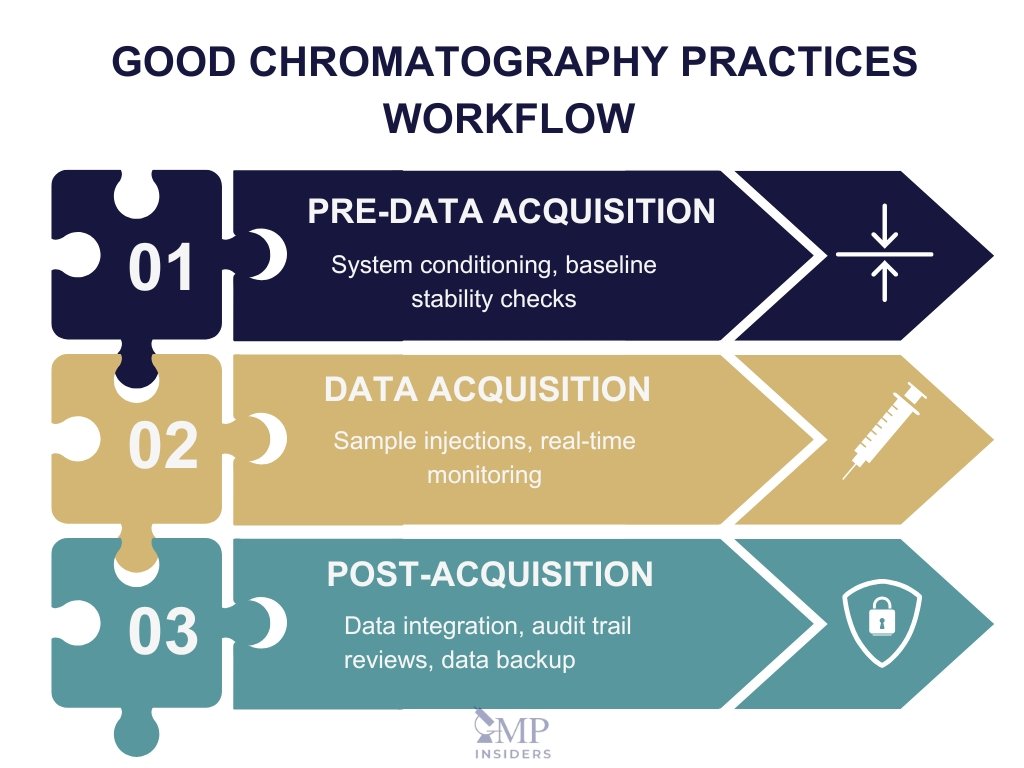
Pre Data Acquisition
Pre-data acquisition steps are essential for ensuring the stability and readiness of the chromatography system. These procedures prevent errors, reduce variability, and ensure that data collected is valid and reproducible.
System Conditioning
Before running samples, the chromatographic system must be properly conditioned to achieve stable baselines and ensure consistent performance throughout the analysis.
Baseline Equilibration
Importance: A stable baseline is essential for accurate peak detection and integration. If the baseline drifts or fluctuates, it can interfere with peak identification and quantitation.
Equilibration Time: Allow the column to equilibrate with the mobile phase until the system reaches a steady state. For gradient elution methods, ensure that the column experiences the full gradient cycle before starting data acquisition.
Monitoring Baseline Stability: Use blank injections or monitor detector signals [Plot] to confirm that the baseline is stable. Only proceed with sample injections after a stable baseline is observed. Some CDS provide automatic “Baseline Check” where data acquisition starts after set baseline parameters are achieved.
Instrument Conditioning: Include flushing mobile phases through the column and system components to remove air bubbles, contaminants, or residue from previous runs.
No Single Run Policy
Definition: A “no single run policy” refers to the practice of avoiding single injections or isolated sample runs outside of a well-structured sequence.
Importance: Isolated runs can result in incomplete system checks and higher risk of inconsistent performance.
Benefit: Running samples as part of a well-planned batch or sequence ensures that system suitability tests (SST) are included, potential carryover is detected with blanks, and the method’s reproducibility is validated.
Data Acquisition
The data acquisition phase is where chromatographic results are generated through structured sample sets (batches or sequences). Proper planning and structuring of sample sets ensures consistent results and prevents issues like carryover, contamination, or missed data points.
Sample Sets (Batches/Sequences)
Sample sets or sequences are predefined groups of samples that are processed in a single chromatographic run to ensure consistency in data acquisition. The organization of these sequences determines the relevance and quality of the acquired data.
Weighing Values for Relevance Before Acquisition
Pre-Acquisition Weighing: Accurate weighing of samples, standards, and controls is critical to ensure the reliability of results. These values are incorporated into calculations, such as concentration and potency determination, during post-acquisition data processing. In order to achieve optimal transparency, it is best to include all weighing and potency factors in sample sets before data acquisition.
Traceability of Weights: Ensure that weighing data is linked to the sample set in the CDS or logbooks to enable traceability and to ensure that any deviations can be investigated effectively.
Use of Template Sample Sets for Routine Use
Consistency: For routine analysis, using template sample sets minimizes the chance of errors by standardizing the sequence structure across multiple batches or runs.
Efficiency: Templates reduce time spent on method setup and ensure that all required samples, standards, and blanks are included consistently.
Flexibility: Templates can be adjusted for specific batches, such as updating product information, batch numbers, or slight modifications to sequences, while still maintaining a standard format.
Structure of Sample Sets
Proper structuring of sample sets ensures smooth data acquisition and helps prevent carryover, contamination, or baseline drift.
Blanks and Placebos
Blanks are run at key points (e.g., at the beginning, between samples, or at the end) to confirm that the chromatographic system is free from contamination or carryover.
Placebo Injections: Placebos are often included in related substances testing to detect interferences from excipients.
Blanks Between Runs (Carryover Prevention): Run blanks between high-concentration samples or standard injections to check for carryover. If peaks are observed in the blank run, the system may need additional flushing or cleaning.
SST Upfront
System Suitability Test (SST) solutions are typically run at the beginning and at the end of a sequence to ensure that the system is functioning properly (e.g., meeting resolution, symmetry, and retention time criteria) during data acquisition. If SST fails, the analysis is halted until the issue is resolved, preventing invalid data acquisition.
Bracketing of Standards
Bracketing involves placing standard injections at intervals throughout the sequence (e.g., at the beginning, middle, and end) to monitor system performance over time.
This approach ensures that any drift in instrument performance or degradation of standards is detected, maintaining the accuracy and validity of results.
Benefits of Bracketing:
Instrument Stability Check: Verifies that retention times and system suitability parameters remain stable throughout the sequence.
Data Quality Assurance: Ensures that results are reliable, especially in long runs where column or detector performance may change over time.
Automated System Suitability Determination
Automation in system suitability determination offers significant benefits, ensuring that only valid runs are completed and reducing the need for manual intervention.
Automated systems can pause sequences or halt runs if SST results fail to meet predefined acceptance criteria. This prevents unnecessary sample consumption and avoids wasting time on invalid data.
Waiting or Reprocessing Commands: If an SST parameter is borderline, automated systems can pause the sequence and wait for further instructions (e.g., system flushing, re-injection of SST solution) before proceeding with the run.
Benefits of Automated SST Systems:
Error Reduction: Automated checks eliminate the possibility of human error in deciding whether SST parameters are met.
Efficient Resource Management: Prevents invalid data acquisition by halting sequences when system suitability criteria are not met.
Improved Workflow: Enables seamless integration with CDS platforms to trigger corrective actions, such as flushing the system or re-running failed SST injections.
Post Acquisition
The post-acquisition phase involves processing, managing, and reporting chromatographic data to ensure that results are accurate, traceable, and secure. Proper practices in this phase ensure compliance with regulatory expectations (e.g., 21 CFR Part 11) and prevent delays that can affect the decision-making process.
Peak Integration
Accurate peak integration is essential for quantifying and identifying analytes. Although automated integration is the preferred approach, there are situations where manual integration may be necessary. However, any manual intervention must be scientifically justified and documented in audit trail to maintain data integrity.
Automated Integration
Automated peak integration uses predefined parameters such as baseline threshold, peak width, and smoothing algorithms to ensure consistency across runs/sample sets.
It eliminates human bias, reduces variability, and ensures reproducibility in large data sets. Automated integration is particularly useful for routine analysis or high-throughput laboratories.
Manual Integration Exceptions
In some cases, baseline drift, overlapping peaks, peak tailing, or noisy signals may require manual adjustments to peak integration.
Justification and Documentation: Every manual change must be scientifically justified and documented with details such as:
- Reason for the change (e.g., shifting baseline, peak splitting)
- User ID and time of adjustment
- An audit trail to track changes, ensuring transparency for future audits
Best Practices:
- Define strict criteria for when manual integration is allowed, documented in Standard Operating Procedures (SOPs).
- Use CDS systems that highlight manual changes for easy identification during review and approval.
Data Management
Proper data management ensures that acquired chromatographic data is processed, stored, and accessed in a way that maintains integrity, security, and traceability.
Timely Data Processing
Data should be processed as soon as possible after acquisition to prevent backlogs or missed deadlines, particularly in regulated environments.
Long delays between data acquisition and analysis may result in missed deviations or critical errors going unnoticed. Laboratories should define turnaround times for data review and processing in SOPs.
Controlled Access to Data Locations
Store data in validated electronic systems (e.g., CDS platforms) with role-based access control to ensure that only authorized users can view, modify, or approve the data.
Implement read-only access for users who only need to view data but do not have permission to modify it, reducing the risk of unauthorized changes.
Data Backup and Retention
Regularly back up chromatographic data according to the lab’s data retention policies. Ensure that backups are stored in secure locations, both onsite and offsite, to prevent data loss due to system failures or disasters.
Data Reporting
Accurate and traceable reporting is crucial to ensure that chromatographic results are audit-ready and meet regulatory requirements. Data reporting must include comprehensive metadata to maintain full traceability.
Key Elements in Chromatographic Reports
Date and Time of Acquisition and Processing: Ensure each report records the exact date and time the data was acquired and processed.
User Identification: The report should include user names or IDs to show who performed specific actions (e.g., acquisition, processing, or approval).
Data Path and Batch Information: Record the data storage path or file location to ensure that results are traceable back to their original data source. Include batch numbers and sample IDs for easy linkage to production or testing records.
Audit Trail Inclusion: Reports must include audit trails where applicable, documenting any changes made during data processing and the corresponding justifications.
Report Approval and Review Process
Reports should follow a multi-level approval process, with different roles (e.g., analyst, reviewer, quality approver) reviewing the data to ensure accuracy.
If electronic signatures are used, ensure compliance with 21 CFR Part 11 requirements, where signatures are linked to specific users and timestamps.
FAQ: Good Chromatography Practices
What Should I Do if My System Suitability Test (Sst) Fails Mid-Sequence?
If your SST fails, first pause the sequence to avoid wasting samples and time. Check the SST parameters that did not meet acceptance criteria (e.g., retention time, peak resolution, or asymmetry).
Troubleshooting steps include verifying column conditions (e.g., temperature, pressure), flushing the system with appropriate solvents, and checking for contamination.
If the issue persists, consider re-calibrating the instrument or adjusting the method parameters based on historical SST data. Some automated systems allow restarting the sequence from the point of failure once the problem is resolved.
What Is the Most Common Cause of Carryover, and How Can I Avoid It?
Carryover often occurs due to incomplete flushing of the injection needle, autosampler, or column between runs—especially with sticky or high-concentration analytes.
To avoid carryover, flush the autosampler with a suitable wash solution and ensure blanks are run between critical samples.
Using guard columns or cleaning the column regularly can also help. For persistent carryover, try increasing the injection wash volume or switching to a more aggressive solvent system.
How Often Should I Replace My Guard Column?
The replacement frequency depends on the sample type, injection volume, and matrix composition. Monitor parameters like system backpressure and resolution to detect any degradation in performance.
As a general rule, replace the guard column every 100–200 injections or when performance issues like increased backpressure are observed.
How Can I Prevent Baseline Drift During Long Runs?
Baseline drift can result from fluctuations in temperature, mobile phase composition, or detector stability. To minimize drift, ensure that the column temperature remains stable and the mobile phase is properly degassed to remove air bubbles.
Use high-purity solvents to avoid contamination, and equilibrate the system thoroughly before starting the run. For gradient methods, confirm that the gradient profile is correctly programmed and consistent across runs.
Can I Reuse Mobile Phases Across Multiple Runs?
While it is technically possible to reuse mobile phases, doing so increases the risk of contamination or degradation, especially if buffers or salts are involved.
If reusing mobile phases, ensure they are filtered and stored in sealed containers to prevent contamination. However, always check for clarity and pH stability before reusing. In regulated environments, fresh mobile phases are generally preferred to ensure compliance and data integrity.
How Do I Determine the Number of Injections a Column Can Handle Before It Needs Replacement?
The lifespan of a column depends on the sample matrix, flow rate, and solvent system. Monitor theoretical plate numbers, resolution, and pressure trends regularly to assess column health.
Some manufacturers provide guidelines on maximum injections, but a common rule is 300–500 injections for standard HPLC columns, depending on use conditions. Using dedicated columns for specific products can also extend column life.
What Should I Do if My System’s Backpressure Suddenly Increases?
A sudden increase in backpressure often indicates a blockage in the system—typically in the column, guard column, or tubing.
Check the guard column first and replace it if necessary. If the issue persists, flush the column with compatible solvents in the direction of normal flow or, if applicable, perform a reverse flush.
Also, inspect the filters and frits for clogging, and ensure the mobile phase is free from particulate contamination.
Conclusion
Good chromatography practices form the foundation of reliable and reproducible analytical results, ensuring that data generated from chromatographic systems is trustworthy and compliant with regulatory standards. Each phase—from system conditioning to data reporting—plays a crucial role in maintaining the integrity and quality of results.
Effective management of columns, reagents, and samples minimizes variability and contamination, while structured methods, controlled access, and bracketing strategies ensure consistency and traceability. Adhering to data integrity principles such as ALCOA++, supported by audit trails, electronic signatures, and secure user access, ensures that data remains compliant and defensible during audits or investigations.
With automation, lifecycle management, and backup strategies becoming increasingly essential, laboratories can minimize errors, improve operational efficiency, and maintain regulatory compliance. Ultimately, good chromatography practices go beyond accuracy—they reflect a commitment to continuous improvement, ensuring product quality and patient safety.















