Historically, the word granulated comes from the Latin word granulatum which means to grain. For millennia, people have manually rolled medicinal powder into pills by rolling it in sugar or honey. Today, that practice evolved into granulation technology that is broadly used in many industries, including the pharmaceutical industry.
By definition, granulation is the process of particle enlargement using the agglomeration technique and is commonly used in the manufacturing of solid dosage forms (capsules or tablets). The development of pharmaceutical granulation began with W. Brockedon’s invention of the tablet press in 1843.
In the 1970s, the introduction of high-speed tablet and capsule-filling machines with automated controls increased the demands on granulation properties. As regulatory requirements became more stringent, particularly for low-dose products, it became essential to develop technologies that ensure the uniformity and quality of granules. These high-speed machines require a consistent material flow to produce reliable pharmaceutical dosage forms.
This article will focus on the granulation process in the pharma industry.
What Is Granulation? Basic Principles and Objectives
Granulation has long been considered a pharmaceutical process of particle design where fine particles are changed to larger ones as granules or agglomerates.
The process converts fine powders into free-flowing, dust-free granules that are easier to compress. However, this method presents challenges due to the high-quality requirements for granules, including content uniformity and specific physicochemical properties like size, bulk density, porosity, hardness, moisture content, and compressibility, along with the physical and chemical stability of the drug.
The process’s primary goals are to decrease dustiness, increase flow ability, increase material density, minimize weight variation in tablets, and increase dissolution rate.
Pharmaceutical granules typically range in size from 0.2 to 4.0 mm, depending on their intended use.
Granulation Equipment
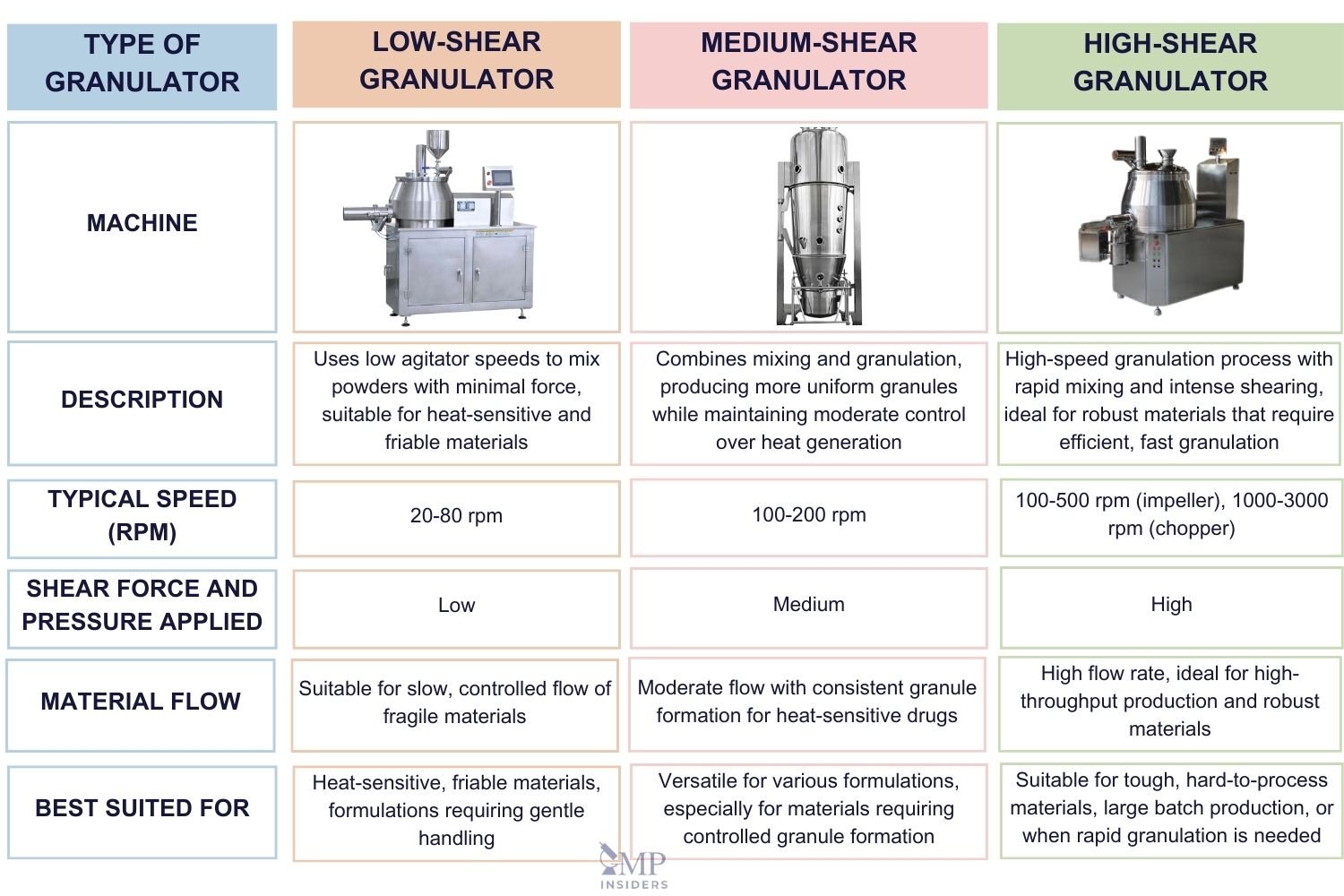
Granulation equipment is categorized into three major types based on the level of shearing force they apply to the powder bed during the granulation process:
Low-Shear Granulators
Low-shear granulators apply less shear force compared to medium- or high-shear granulators, due to their lower agitator speeds, sweep volumes, or bed pressures on the powder bed. Examples of low-shear granulators include: ribbon and paddle blenders, planetary mixer granulators, orbiting screw granulators, sigma blade mixers, and rotating-shape mixers/granulators.
Medium-Shear Granulators
Medium-shear granulators are essential equipment in the pharmaceutical industry, particularly for wet granulation processes in tablet manufacturing. These granulators combine mixing and granulation into a single process, using medium levels of shear force to produce uniform, well-formed granules.
The shear forces are carefully controlled to avoid excessive heat or energy input, making medium-shear granulators suitable for heat-sensitive materials. One of their key advantages is the ability to control particle size distribution effectively, resulting in consistent granule formation, which improves the flow properties and compressibility of the material for further processing.
Additionally, these granulators are versatile and capable of handling a wide range of formulations, making them an ideal choice for producing high-quality granules efficiently while maintaining the integrity of the active pharmaceutical ingredients.
This flexibility allows manufacturers to optimize granule characteristics for different drug formulations, enhancing overall product performance. Furthermore, medium-shear granulators with specialized attachments, like the rotogranulator, offer greater precision and control over the granulation process, ensuring uniformity and consistency across large production batches.
Fluid Bed
Fluid bed granulation is a process where fine solids are transformed into a fluid-like state by contact with a gas, typically air. At a specific gas velocity, the gas supports the particles, allowing them to move freely without becoming trapped.
In this process, granules are formed within a single piece of equipment by spraying a binder solution onto a fluidized powder bed. The system works by heating air and directing it through the material being processed. The heated air flows through the voids in the material, allowing the granules to form and dry simultaneously.
High-shear granulators
Conventional high-shear granulators typically feature a mixing bowl, a three-bladed impeller, and an auxiliary chopper. The mixing bowl can be either cylindrical or conical and may be jacketed to allow for the heating or cooling of the contents by circulating hot or cool liquids or steam through the jacket.
The impeller is used to mix the dry powder and distribute the granulating fluid evenly. In a high-shear granulator, the impeller typically rotates at speeds of 100 to 500 rpm, while the chopper operates at speeds ranging from 1000 to 3000 rpm to break down wet lumps into granules.
High-shear granulators can be classified as either vertical or horizontal, depending on the orientation and positioning of the impeller. Vertical high-shear granulators can be further categorized as top-driven or bottom-driven units.
Role of Granulation in Improving Compressibility and Flow Properties
In pharmaceutical powder compaction, enhancing material flow properties is often necessary to achieve uniform die-filling in a tablet press. This can be accomplished by converting fine powders into larger agglomerates, which improve the flow characteristics of the material.
Compressibility
Improving compressibility through granulation involves altering the physical characteristics of powders by agglomerating fine particles into larger, more cohesive granules. This process enhances compressibility in several ways:
Granulation increases particle size, making granules larger and more uniform compared to individual powder particles. This larger size improves the material’s ability to be compressed into tablets with consistent hardness and density.
The introduction of binding agents during granulation enhances cohesion among the powder particles. This increased cohesion makes the powder mixture more amenable to compression, thereby reducing the likelihood of defects in the final tablets.
Lastly, granulation reduces the segregation of powder components by creating a more homogeneous mixture. This uniformity in granule composition leads to more consistent compressibility and more reliable tablet formation.
Flow Properties
Powder flowability is crucial for ensuring the consistent delivery of powder from the hopper to the die during the tableting process. Inconsistent powder flow can lead to significant variations in tablet weight, which is unacceptable in pharmaceutical production.
Additionally, uneven flow may trap excess air within the powder, increasing the risk of capping or lamination, especially under high-speed tableting conditions. Active pharmaceutical ingredients like acetaminophen or ibuprofen, for example, have poor flow and compression properties and are required in relatively high doses, are often granulated before being compressed into tablets.
This is because the main purpose of granulation is to enhance the flowability and compressibility of the powder mixture.
Poorly flowing powders also struggle to move efficiently within the granulation chamber, negatively impacting the granulation process and the quality of the granules produced.
Therefore, understanding the flowability of powders, particularly bulk excipients, is essential to prevent processing issues. Tools like the Hausner ratio, Carr index, and angle of repose are used to measure and evaluate powder flowability, helping to identify potential problems and implement corrective actions.
The Hausner ratio is a measure used to assess the flowability of a powder by comparing its tapped density to its bulk density. It is calculated by dividing the tapped density by the bulk density. A Hausner ratio below 1.25 indicates good flowability, while a ratio above 1.25 suggests poor flow properties.
The Carr index, or compressibility index, is another measure of powder flow. It is determined by the difference between tapped and bulk density, divided by tapped density, and multiplied by 100. A Carr index value below 15% indicates excellent flowability, while values above 25% reflect poor flow characteristics.
The angle of repose is the steepest angle at which a heap of powder remains stable without sliding. It is an indicator of how well a powder flows. An angle below 30° suggests excellent flow properties, whereas an angle above 40° indicates poor flowability.
SEE ALSO: Powders vs Granules vs Tablets in GMP
Granulation Methods in Pharma Manufacturing
Granulation is typically divided into three commonly used types: wet granulation, which uses a liquid, dry granulation, which does not and hot-melt granulation. Selecting the appropriate process depends on a thorough understanding of the drug’s physicochemical properties, excipients, and desired flow and release characteristics.
Wet Granulation
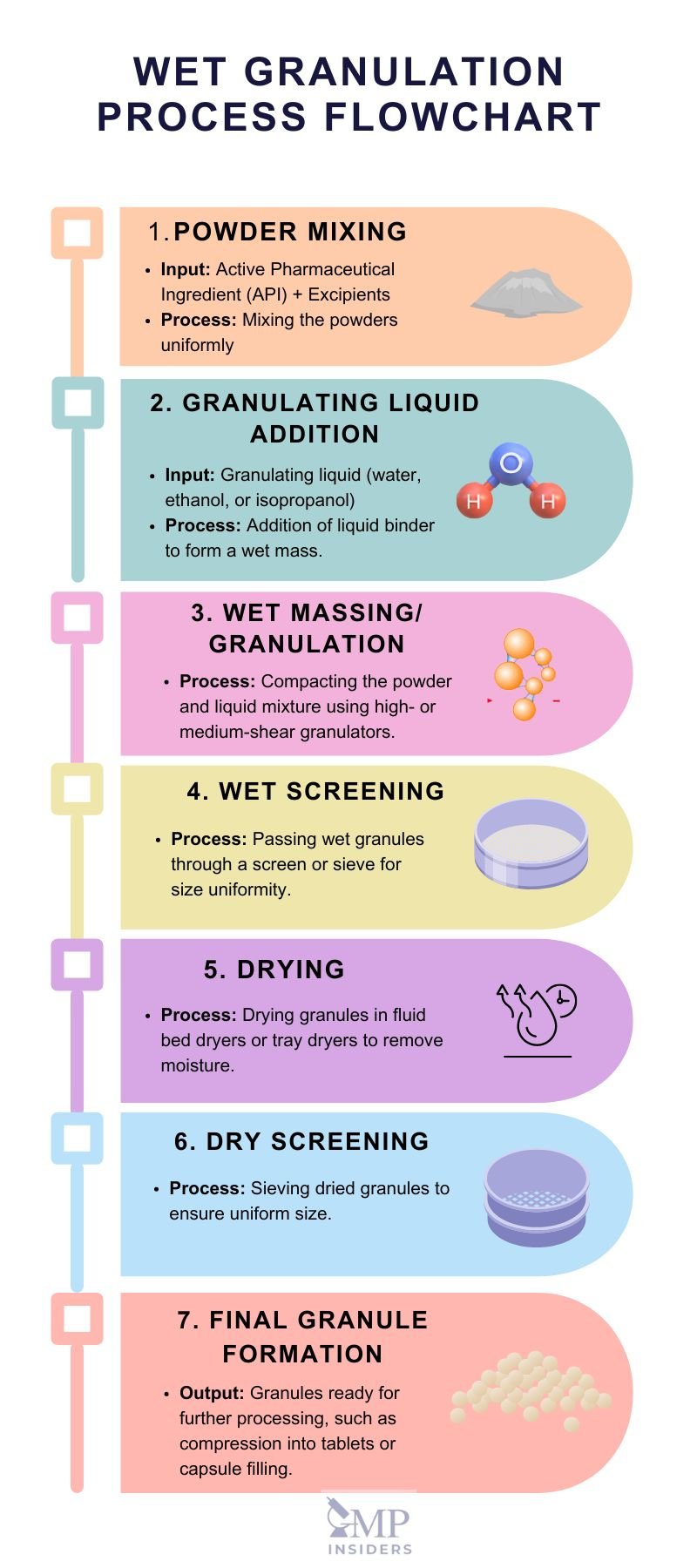
This process involves massing a mixture of dry primary powder particles using a granulating fluid, which is the addition of a liquid solution to the powders. The fluid contains a solvent that must be volatile, allowing it to be removed by drying, and non-toxic to ensure safety.
Typical granulating liquids include water, ethanol, and isopropanol, either used alone or in combination. Often wet granulation involves high-shear granulation, low-shear granulation, and fluid bed granulation.
Recent wet granulation methods include:
- Steam granulation– water steam is used as the binder instead of traditional liquid water. This method offers an alternative to conventional granulation liquids
- Reverse wet granulation – This process involves immersing the dry powder formulation into the binder liquid, followed by controlled breakage to form granules. Initially, the binder solution is prepared, and then the dry powder excipients are added to the solution while mixing in a granulator.
- Moisture-Activated Dry Granulation (MADG)In Moisture-Activated Dry Granulation (MADG), a minimal amount of liquid is used to activate a binder in a planetary mixer. After the binder is activated, any excess moisture is absorbed by adding a moisture-absorbing substance. This technique allows for the formation of granules without the need for extensive drying.
- Thermal Adhesion Granulation (TAG) is similar to moist granulation, involving the addition of a small amount of granulation liquid combined with heat to promote agglomeration. This process can use either water or a solvent as the granulation liquid. Heat is applied to facilitate the granulation process, with the drug and excipient mixture being heated to a temperature of 30-130°C in a closed system under tumble rotation. This helps the powder particles agglomerate effectively.
Dry Granulation
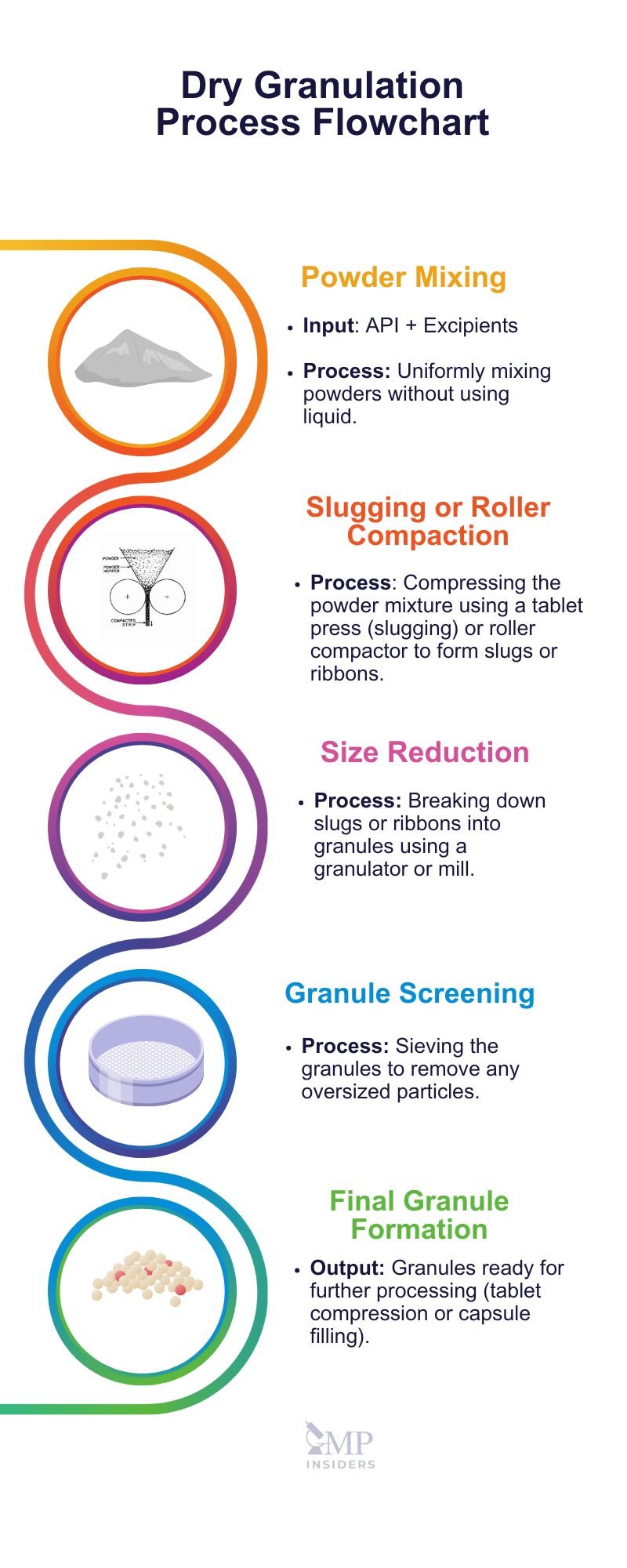
This method is used to form granules without the use of a liquid solution, particularly when the product to be granulated is sensitive to moisture and heat. Advantages include low cost and simplicity.
In the dry granulation method, primary powder particles are aggregated under high pressure. There are two main processes:
Slugging
The slugging process is a dry granulation technique that forms granules without the use of liquid binders. It begins with preparing and mixing the primary powder material. This powder is then fed into a heavy-duty tableting press, which compresses it into large tablets known as slugs. These slugs are subjected to high pressure, causing the powder particles to bond together.
Once the slugs are formed, they are broken down into smaller granules using a granulator or mill to achieve the desired granule size. The granules are then sifted to remove oversized particles and ensure uniformity.
One advantage of the slugging process is that it does not require liquid binders, making it suitable for heat-sensitive or moisture-sensitive materials. Additionally, it can be efficient for certain powders that are challenging to granulate with other methods. However, the process may involve high initial equipment costs and might not be suitable for all types of powders, particularly those requiring very fine granules or specific properties.
Roller Compaction
Roller compaction is a dry granulation technique used to produce granules without the use of liquid binders. In this process, the powder material is fed into a roller compactor, which consists of two counter-rotating rollers that compress the powder between them.
The process begins with the powder being fed into the gap between the rollers. As the rollers rotate, they apply high pressure to the powder, causing it to compact into a dense sheet or ribbon. This sheet is then broken down into granules using a granulator or mill. The granules are subsequently sifted to ensure uniform size and to remove any oversized particles.
Roller compaction is advantageous because it avoids the use of moisture or heat, making it suitable for sensitive materials that may be affected by these factors. It is also efficient for producing granules with a consistent size and density. However, the equipment can be expensive, and the process may not be suitable for all types of powders, particularly those that do not compact well or require specific properties for the final product.
There has been limited progress in dry granulation technology compared to wet granulation. However, a notable innovation in this field is pneumatic dry granulation technology, developed by Atacama Labs Oy in Helsinki, Finland. This advancement represents a significant development in the dry granulation process.
Melt Granulation
Melt granulation is a size enlargement process where a binder that melts or softens at relatively low temperatures (around 60°C) is used to agglomerate solid particles in a formulation. This technique employs materials that act as effective granulating agents in their softened or molten state.
Melt granulation is particularly useful for preparing sustained-release dosage forms using lipophilic polymers. This method is less time-consuming and more energy-efficient compared to wet granulation. In addition to the previous statement, melt granulation is great for water-sensitive materials.
Freeze Granulation
Freeze granulation involves spraying droplets of a liquid slurry or suspension into liquid nitrogen, causing the droplets to freeze instantly. The frozen droplets are then freeze-dried, where the ice sublimates, leaving behind dry granules. This method efficiently creates granules by first solidifying the droplets in liquid nitrogen and then removing the ice through freeze-drying.
Continuous Granulation in Modern Manufacturing
Continuous granulation is a modern manufacturing technique that represents a significant advancement over traditional batch processes. It involves the continuous production of granules, offering several benefits in terms of efficiency and product quality.
In continuous granulation, raw materials, including powders and binders, are continuously fed into the granulator. This steady feed system allows for uninterrupted processing throughout the operation. The materials are processed in the granulator, where they are subjected to mechanical forces, heat, and/or a liquid binder, depending on whether wet or dry granulation is used.
As the process progresses, the granules are continuously formed from the powder mixture. In the case of wet granulation, the granules are then dried continuously using integrated drying equipment such as fluid bed dryers.
After drying, the granules are cooled to stabilize them. The finished granules are screened to ensure uniform size and to remove any oversized particles before being packaged or sent for further processing.
The advantages of continuous granulation include increased efficiency due to the elimination of batch-to-batch transfers, improved consistency in granule size and quality, and the capability for real-time monitoring and control. The continuous nature of the process reduces overall processing time and facilitates easier scaling from pilot to full-scale production.
Continuous granulation is widely used in pharmaceuticals for producing tablets and capsules with consistent quality and controlled release properties, in nutraceuticals for dietary supplements, and in chemicals for various powder and granule products.
However, the technique can be more complex and costly to set up and maintain compared to traditional batch equipment, and it requires sophisticated control systems to manage the continuous flow of materials and ensure consistent product quality.
Challenges in Granulation
Granulation, while essential for enhancing the properties of powders, presents several challenges that can impact the effectiveness of the process and the quality of the final product.
1. One major challenge is achieving a consistent particle size distribution. Variability in granule size can lead to inconsistent flow and compression properties, affecting the quality of the final product. Controlling the particle size during granulation requires careful adjustment of process parameters and equipment settings.
Pro Tip: Regularly adjust process parameters like mixer speed and granulation time to control particle size.
2. Moisture control is another critical issue, especially in wet granulation. Managing moisture levels accurately is crucial because too much moisture can cause over-wetting, leading to clumping or inconsistent drying, while too little moisture may result in insufficient granulation and poor granule formation.
Pro Tip: Fine-tune moisture levels by closely monitoring the amount of liquid added and the drying process to avoid clumping or poor granule formation.
3. The selection and optimization of binders are also vital. The type and concentration of binders used significantly impact the granulation process. An incorrect binder or concentration can affect granule strength, dissolution rate, and overall product performance.
Pro Tip: Choose binders carefully based on the desired granule strength and dissolution rate. Test different concentrations to find the optimal amount.
4. Maintaining and calibrating granulation equipment, such as granulators, dryers, and mixers, is essential to ensure consistent performance. Equipment malfunctions or improper calibration can lead to variations in granule size, density, and quality.
Pro Tip: Maintain and calibrate granulators, dryers, and mixers regularly to prevent performance variations and ensure granule quality.
5. Scaling up from laboratory or pilot-scale granulation to full production scale can be challenging due to differences in equipment and process conditions. Ensuring that the process remains consistent during scale-up requires thorough validation and adjustments.
Pro Tip: Validate the granulation process during scale-up by adjusting process conditions to match the full production scale and ensure consistency.
SEE MORE: Process Validation In GMP
6. Effective process control and monitoring are crucial for maintaining precise control over granulation parameters, such as speed, temperature, and mixing time. Implementing effective systems helps detect and correct deviations in real-time.
Pro Tip: Implement real-time monitoring systems for critical parameters like speed and temperature to detect and correct deviations promptly.
7. Compatibility of ingredients is also a concern. Ensuring that active pharmaceutical ingredients (APIs) and excipients are compatible is crucial, as adverse interactions can affect the granulation process and the stability of the final product.
Pro Tip: Assess API and excipient compatibility early in development to avoid adverse interactions that could impact granulation and product stability.
8. Granule strength and stability are important for the final product’s performance. Granules must be able to withstand handling, compression, and storage without degrading or losing their integrity.
Pro Tip: Test granules for mechanical strength and stability under various conditions to ensure they withstand handling and compression.
Addressing these challenges requires a thorough understanding of the granulation process, careful selection of materials and equipment, and diligent process control to ensure the production of high-quality granules and final products.
FAQ
What Is the Difference Between Granulation and Powder?
Powder consists of fine, loose particles that can be difficult to handle due to poor flowability and compressibility. Granulation involves forming these powders into larger, cohesive granules, improving flowability, compressibility, and reducing dust, which enhances handling and manufacturing efficiency.
Which Is Better Dry or Wet Granulation?
The choice between dry granulation and wet granulation depends on the specific needs of the formulation. Wet granulation is preferred for formulations requiring improved flowability and uniformity, producing stronger and more consistent granules but involving a time-consuming drying step. Dry granulation is suitable for heat- or moisture-sensitive drugs, as it eliminates the need for liquid and drying, though it may produce weaker granules and requires specialized equipment.
What Is the End Point of Granulation?
The endpoint of granulation refers to the stage in the granulation process where the desired granule properties, such as size, moisture content, and consistency, are achieved. It is the point where further granulation is no longer necessary, and continuing the process could degrade the quality of the granules. Reaching the end point ensures optimal granule formation for efficient processing, such as tableting or encapsulation.
Why Were Granules Preferred Over Powder in the Manufacturing of Tablets?
Granules are preferred over powders in tablet manufacturing because they offer improved flowability and compressibility, leading to more consistent tablet weight and strength. Granules also produce less dust, reducing material loss and safety risks, and they enhance content uniformity and reduce the risk of segregation, resulting in higher-quality tablets.
What Is the Difference Between Granules and Tablets?
Granules are small aggregates of powder used to improve flowability and compressibility, often serving as an intermediate step in tablet production. Tablets are solid dosage forms made by compressing granules or powders into a specific shape, designed to deliver a precise dose of medication to patients.
Conclusion
Granulation is a vital process in pharmaceutical manufacturing that significantly enhances the properties of powders, including their flowability, compressibility, and uniformity. By converting fine powders into larger, more cohesive granules, granulation improves the consistency and quality of solid dosage forms like tablets and capsules.
Despite its critical role, granulation presents various challenges, such as maintaining particle size distribution, managing moisture levels, and optimizing binder use. Advances in granulation technology, such as continuous granulation and innovative methods like steam and melt granulation, offer promising solutions to these challenges, leading to more efficient and reliable production processes.