For decades, High-Performance Liquid Chromatography (HPLC) has been the trusted analytical workhorse, known for its robustness, reproducibility, and widespread inclusion in nearly every pharmacopeial monograph. Yet, as laboratories face increasing pressure to accelerate analysis, reduce solvent use, and detect impurities at lower levels, the shift toward Ultra-Performance Liquid Chromatography (UPLC) has become inevitable.
Although both techniques rely on the same separation principles, they differ fundamentally in column design use, operating pressure, and system configuration. Such technical differences directly influence resolution, runtime, solvent consumption, and the overall cost of analysis, shaping how efficiently laboratories can meet today’s regulatory and productivity demands.
This article provides a detailed comparison of HPLC and UPLC in pharmaceutical practice, focusing on their performance characteristics, compatibility, operational differences, cost implications, and method transfer challenges, to help laboratories decide when to maintain proven HPLC systems and when to adopt UPLC for next-generation analytical performance.
HPLC and UPLC: Definitions and Applications
Both HPLC and UPLC are grounded in the same fundamental principle, the separation of chemical components based on their differential distribution between a mobile phase (a liquid solvent) and a stationary phase (a packed column). As analytes pass through the column, their varying affinities for each phase cause them to elute at distinct retention times, enabling precise identification and quantification.
As analytical demands increased, with the need for shorter runtimes, greater sensitivity, and reduced solvent consumption, conventional HPLC systems reached their practical limits. This technological ceiling led to the development of UPLC, which applies the same chromatographic theory under higher pressures and with smaller column particles to achieve faster, more efficient separations without sacrificing accuracy or compliance.
| Parameter | HPLC | UPLC |
|---|---|---|
| Typical Pressure Range | Up to 400 bar | Up to 1,000–1,200 bar |
| Column Particle Size | 3–5 µm | <2 µm |
| Typical Runtime | 20–45 min | 2–5 min |
| Sensitivity | Moderate | High |
| Solvent Consumption | High | Low |
| Common Use | QC, Routine Analysis | R&D, Method Development |
What Is HPLC?
High-Performance Liquid Chromatography (HPLC) separates analytes based on their interactions between a liquid mobile phase and a solid stationary phase under pressures typically up to 400 bar. Using column particles of 3–5 µm, it provides consistent, high-quality separations suitable for most routine analytical tasks.
In pharmaceutical environments, HPLC remains the benchmark method for quantitative analysis, impurity profiling, and validation of finished products. Its reliability, regulatory acceptance, and compatibility with a wide range of detectors make it an essential tool across development and quality control laboratories.
Applications:
- Pharmaceuticals: Assay and impurity profiling of active substances and finished products.
- Food analysis: Determination of additives, preservatives, and colorants.
- Environmental testing: Quantification of pesticides and organic pollutants.
Clinical and bioanalytical studies: Measurement of drugs and metabolites in biological fluids.
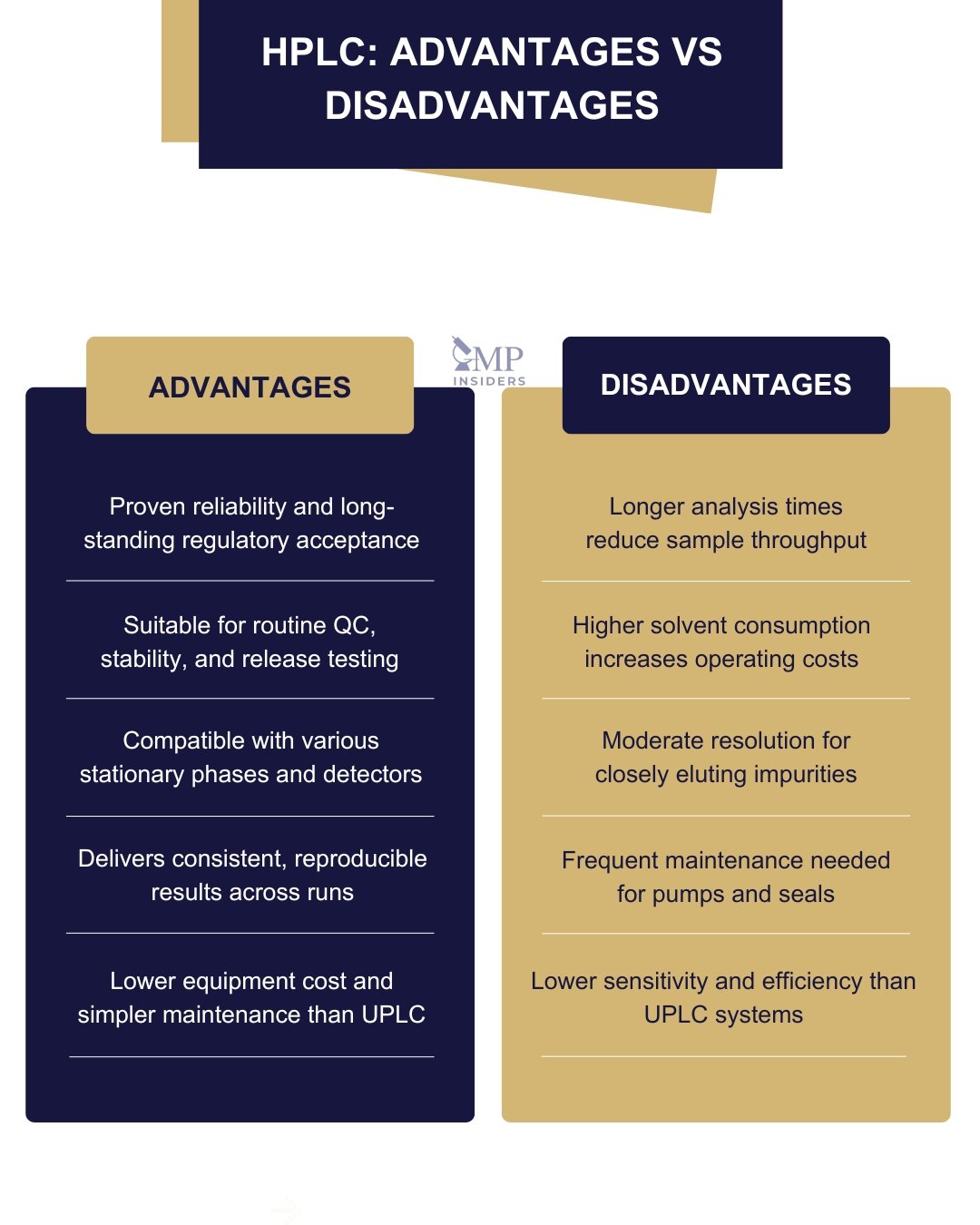
Advantages:
- Proven reliability: Supported by decades of regulatory data and harmonized pharmacopeial methods.
- Versatility: Broad choice of stationary phases, solvents, and detectors (UV, PDA, fluorescence, MS).
- Reproducibility: Stable retention times and method robustness under routine QC conditions.
Limitations:
- Longer analysis times and higher solvent consumption, reducing throughput and increasing operational cost.
- Moderate resolution for closely eluting impurities due to larger particle sizes.
- Frequent maintenance, as mechanical components are subject to continuous high-pressure operation.
While newer systems have surpassed it in speed and efficiency, HPLC continues to serve as the reference platform in regulated testing, the foundation upon which UPLC technology was developed and optimized.
What Is UPLC?
Ultra-Performance Liquid Chromatography (UPLC) evolved from HPLC to meet the growing need for faster analyses, higher resolution, and improved detection sensitivity. The main innovation lies in the use of sub-2 µm stationary phase particles, which significantly increase chromatographic efficiency. To accommodate these smaller particles, UPLC systems operate at pressures exceeding 1,000 bar, several times higher than traditional HPLC instruments.
This combination of higher pressure and smaller particles allows analytes to elute more rapidly with sharper peaks and improved separation between closely related compounds. The result is shorter runtimes, reduced solvent use, and enhanced signal clarity, without compromising reproducibility or quantitation accuracy.
UPLC systems are built with low-dispersion flow paths, high-precision pumps, and minimized dead volumes, ensuring that peak integrity is maintained even under ultra-high-pressure conditions. These design optimizations make UPLC particularly valuable for laboratories where sample throughput and data precision are critical.
Applications:
- Pharmaceutical analysis: Quantification of active substances and impurities with faster turnaround times.
- Biopharmaceuticals: Characterization of peptides, proteins, and glycan structures.
- Environmental and food testing: Detection of trace pollutants in water and soil.
- Metabolomics and research studies: Comprehensive profiling of small molecules within complex matrices.
Advantages:
- Significantly reduced analysis time, most runs are completed within minutes.
- Superior resolution, enabling better separation of complex or closely eluting peaks.
- Lower solvent consumption, contributing to cost reduction and greener operation.
- Enhanced sensitivity, improving detection limits and quantification accuracy.
Limitations:
- Higher system and column costs, driven by the need for specialized high-pressure hardware.
- Shorter column lifetime, as sub-2 µm particles are more prone to fouling.
- Greater sensitivity to particulates, requiring thorough sample filtration and solvent quality control.
- Complex method transfer, since scaling from HPLC demands re-optimization of flow rates and gradient programs.
Rather than replacing HPLC outright, UPLC represents its technological evolution, extending chromatographic capability to deliver faster, cleaner, and more data-rich analyses in modern pharmaceutical and research environments.
HPLC vs. UPLC: Key Differences
Key differences between HPLC and UPLC arise from how each system is engineered and operated. Variations in column geometry, pressure capability, and flow dynamics directly affect resolution, runtime, and method adaptability.
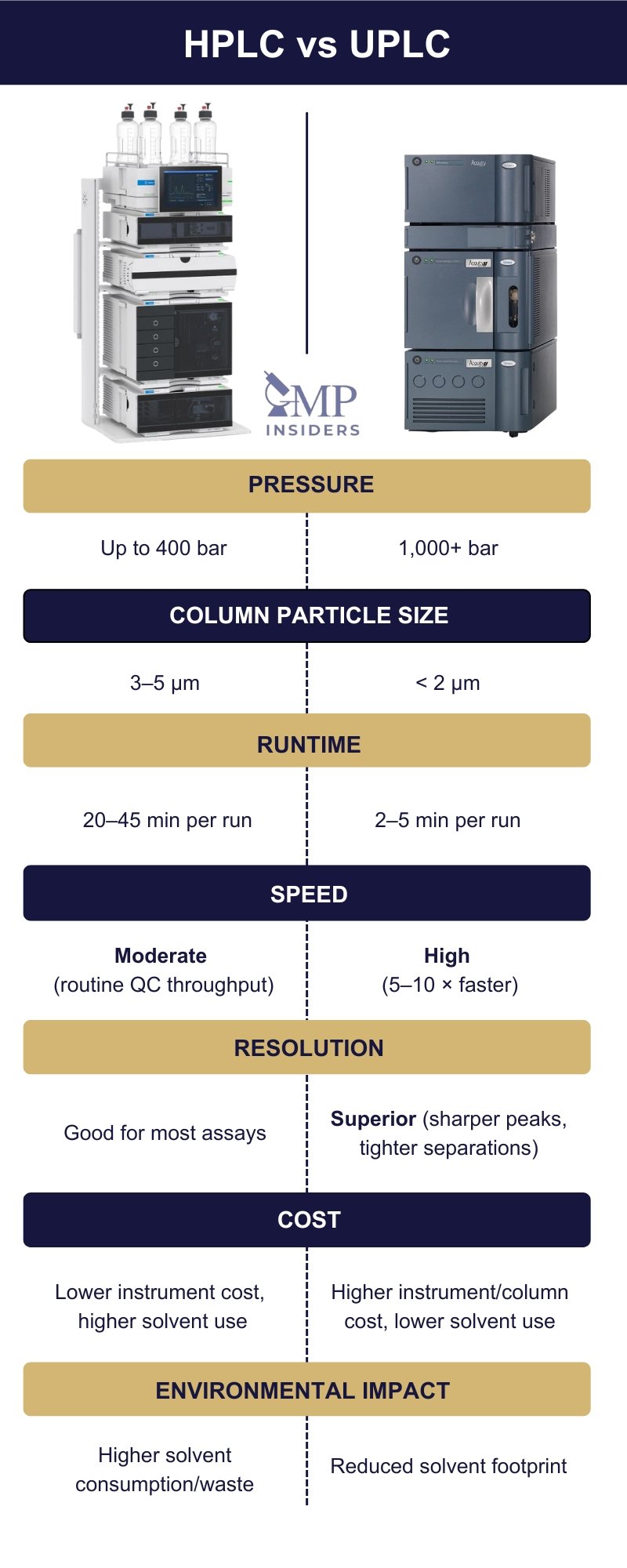
Columns
In HPLC, longer columns (150–250 mm) with 4.6 mm internal diameters (ID) are preferred for robustness and tolerance to higher injection volumes, making them ideal for validated QC methods. UPLC uses much shorter, narrower columns (30–100 mm, 2.1 mm ID) to achieve equivalent or better resolution in a fraction of the time.
The smaller particle size in UPLC (sub-2 µm) delivers higher efficiency but demands greater control over backpressure and system cleanliness. In practice, analysts using UPLC must maintain strict filtration (0.2 µm) and avoid buffer precipitation, while HPLC systems can operate reliably with standard 0.45 µm filtration and less stringent cleaning.
HPLC columns generally last longer and can handle moderate contamination or pressure fluctuations without performance loss. UPLC columns, operating at several times higher pressure, are more prone to fouling or mechanical failure if preventive maintenance and flushing routines are not tightly followed.
Operating Ranges
During implementation, HPLC methods typically run between 0.5–2.0 mL/min and up to 400 bar, producing analysis times around 20–45 minutes. UPLC methods, running at 0.2–0.5 mL/min and pressures above 1,000 bar, shorten runtime to around 2–5 minutes while reducing solvent use by roughly 70–80%.
Temperature control becomes more critical in UPLC: even small variations can influence retention and peak symmetry due to the higher system sensitivity. HPLC methods are generally stable under ±3 °C fluctuation and can often run at ambient temperature without noticeable changes in selectivity.
In UPLC, injection precision and autosampler calibration must be tightly verified; small deviations in volume or timing can distort peak shapes. HPLC is more forgiving, making it better suited for multi-operator QC labs with high sample volume and varied experience levels.
Method Transfer and Performance
When transferring an HPLC method to UPLC, scaling factors must be applied to preserve linear velocity and selectivity. A 250 mm × 4.6 mm HPLC column (5 µm) often translates to a 100 mm × 2.1 mm UPLC column (1.7 µm) with a flow reduction from 1.0 mL/min to approximately 0.3 mL/min. Without scaling gradient time and dwell volume, retention shifts and loss of resolution are common.
UPLC systems also require more precise equilibration steps, as reduced column volume and higher pressure amplify any inconsistency in gradient mixing. Analysts must verify dwell-volume compensation and confirm gradient delay during method transfer.
In practice, UPLC offers distinct performance advantages, faster separations, lower solvent costs, and improved impurity resolution, but demands careful revalidation. HPLC methods, while slower, remain more stable across instruments and analysts, which explains their dominance in compendial and release testing.
| Parameter | HPLC (Example) | UPLC (Scaled) | Adjustment |
|---|---|---|---|
| Column | 250 × 4.6 mm (5 µm) | 100 × 2.1 mm (1.7 µm) | Scale by L×d² |
| Flow Rate | 1.0 mL/min | 0.3 mL/min | Reduce proportionally |
| Injection Volume | 10 µL | 1–2 µL | Scale by column volume |
| Gradient Time | 20 min | 6 min | Adjust for dwell volume |
How to Scale from HPLC to UPLC
- Column volume / geometric scale (r):
r = (L₂ · d₂²) / (L₁ · d₁²)Use this ratio to scale injection volume and gradient time between systems. - Flow rate (match linear velocity):
F₂ = F₁ · (d₂² / d₁²) · (dₚ₁ / dₚ₂)Keeps linear velocity constant. Include the particle-size term (dₚ₁/dₚ₂) if particle sizes differ. - Injection volume (avoid overload):
Vinj,2 = Vinj,1 · rScale by column-volume ratio, but keep Vinj,2 ≤ 1–2 % of column volume to prevent peak broadening. - Gradient time (account for dwell volume):
tG,2 = tG,1 · (r · F₁ / F₂) + (VDw,2/F₂ − VDw,1/F₁)Adjust for system dwell-volume differences to maintain equivalent gradient slope.
HPLC → 250 × 4.6 mm, F₁ = 1.0 mL/min
UPLC → 100 × 2.1 mm, target F₂ ≈ 0.30 mL/min
- r = (100 · 2.1²)/(250 · 4.6²) ≈ 0.083
- Flow (by d²): F₂ = 1.0 · (2.1²/4.6²) ≈ 0.21 mL/min → practical setting 0.30 mL/min
- Injection: Vinj,2 = 10 µL · 0.083 ≈ 0.8 µL → use 1–2 µL
- Gradient: tG,2 ≈ 20 min · (0.083 · 1.0 / 0.30) ≈ 5.5–6.0 min
- L — Column length (mm)
- d — Internal diameter (mm)
- dₚ — Particle diameter (µm)
- F — Flow rate (mL/min)
- r — Scaling ratio (volume/geometric factor)
- Vinj — Injection volume (µL)
- VDw — Dwell volume (mL)
- tG — Gradient time (min)
- ₁ — HPLC system parameters
- ₂ — UPLC system parameters
SEE ALSO: Performance Characteristics in Method Validation
Operational Considerations
While HPLC and UPLC are built on the same chromatographic principles, their operational demands differ significantly. These differences influence daily laboratory performance, maintenance routines, and training requirements, factors that often determine whether a laboratory remains with HPLC or transitions to UPLC.
Sample Preparation
HPLC systems are generally more tolerant of sample variability and particulate matter, allowing for simpler preparation steps such as dilution and filtration through 0.45 µm membranes.
In contrast, UPLC columns, which use sub-2 µm particles and smaller internal diameters, are more prone to clogging. They require stricter filtration (typically 0.2 µm or finer) and careful solvent quality control to prevent pressure spikes and baseline noise. Even minor inconsistencies in sample cleanliness can lead to premature column fouling or retention time shifts.
System Maintenance
The higher pressure and tighter tolerances of UPLC systems demand more frequent and precise maintenance. Tubing, seals, and check valves experience greater mechanical stress, and column lifetimes are shorter if cleaning or flushing routines are not consistently followed.
HPLC systems, by comparison, are more forgiving and less expensive to maintain. Their longer column lifespans and lower pressure stress make them well-suited for long-term routine testing and validated QC methods.
Ease of Use and Training
HPLC instruments are widely used, and most analysts are already trained in their operation and troubleshooting. UPLC systems, however, require a deeper understanding of method optimization, scaling, and gradient control. Minor deviations in flow rate or injection volume have a proportionally larger effect on chromatographic performance, so operator competence plays a greater role in reproducibility.
SEE ALSO: Good Chromatography Practices as Industry Standards
Throughput and Resource Use
The operational efficiency of UPLC is its main practical advantage. Shorter runtimes and reduced solvent consumption increase sample throughput and support sustainability initiatives. HPLC, though slower, remains advantageous in regulated QC environments where method consistency and historical data take precedence over speed.
Overall, laboratories selecting between HPLC and UPLC must balance analytical performance, resource efficiency, and method robustness. In most cases, HPLC remains the preferred platform for validated, routine testing, while UPLC offers clear benefits for high-throughput environments and advanced analytical development.
HPLC vs UPLC – Similarities
Despite their differences in performance and hardware, HPLC and UPLC are built on the same chromatographic foundation. Both techniques rely on the interaction between analytes and a stationary phase as a liquid mobile phase passes through the column. The mechanisms governing retention, selectivity, and separation efficiency remain identical; what differs is the scale and optimization of these parameters.
Because the theoretical basis is shared, method transfer between HPLC and UPLC is often feasible when the same stationary phase chemistry and comparable gradient conditions are maintained. Columns in both systems often use similar bonded phases, such as C18, C8, phenyl, cyano, or HILIC, ensuring that selectivity and elution order are generally preserved.
The instrument architecture also follows the same structure: a pump to deliver the mobile phase, an autosampler for precise injection, a column for separation, a detector for quantitation, and a data system for integration and reporting. This structural alignment allows analytical scientists to adapt familiar workflows, even as system capability advances.
Detection techniques remain consistent across both systems, using photodiode array (PDA), fluorescence, or mass spectrometry (MS), depending on the analytical objective. As a result, data interpretation, calibration models, and validation strategies developed for HPLC often remain applicable in UPLC workflows.
In essence, UPLC extends the same chromatographic principles to a higher level of performance, offering greater efficiency and speed while retaining the theoretical and procedural continuity that makes HPLC methods so well-established in pharmaceutical analysis.
Compatibility and Limitations
Integrating HPLC and UPLC systems within the same laboratory often presents practical challenges. While both techniques share the same theoretical foundation, their hardware tolerances, flow paths, and detector volumes are not interchangeable, meaning cross-use of columns or methods requires careful consideration.

UPLC Columns on HPLC Systems
UPLC columns are not compatible with standard HPLC instruments. Their sub-2 µm packing generates backpressures that can easily exceed the mechanical limits of HPLC pumps and seals. Attempting to use them on conventional systems can lead to pressure overload, unstable flow, or permanent damage to the pump heads and check valves. Even when pressure limits are not immediately reached, flow inconsistencies and retention drift make results unreliable.
HPLC Columns on UPLC Systems
HPLC columns, on the other hand, can be operated safely on UPLC instruments, as the pressure requirement is well below system capability. However, the chromatographic performance remains identical to that of standard HPLC, longer run times, broader peaks, and higher solvent consumption. In practice, using HPLC columns on UPLC systems is only useful when verifying legacy methods or maintaining continuity during instrument replacement.
System Volume and Detector Mismatch
The flow path and detector cell volumes differ substantially between the two systems. UPLC instruments are optimized for low-dispersion operation, while HPLC detectors and tubing have larger internal diameters. When mismatched, this leads to peak broadening, tailing, or distorted resolution. For example, connecting a UPLC column to an HPLC detector introduces excessive dead volume, negating the performance advantage of the smaller column.
Hybrid Solutions
Many laboratories bridge the gap using core–shell (superficially porous) columns with 2.6 µm particles. These columns provide UPLC-like efficiency but operate at HPLC pressures, allowing use on most modern HPLC systems without hardware upgrades. Core–shell technology is particularly useful for analytical development teams that need improved resolution or faster throughput while maintaining validated HPLC infrastructure.
In practical terms, full interchangeability between HPLC and UPLC hardware is not feasible. Each system must be operated within its own mechanical and flow-path limitations, with column selection and method parameters carefully matched to system design.
HPLC vs UPLC: Cost Implications
The financial impact of choosing between HPLC and UPLC extends beyond the initial purchase price. Cost differences emerge through solvent consumption, column lifespan, maintenance frequency, and analytical throughput, all of which vary depending on how each system is used in practice.
| Cost Factor | HPLC | UPLC |
|---|---|---|
| Initial Investment | Lower; widely available systems suitable for most QC and analytical labs. | Higher; advanced hardware required to handle ultra-high pressures. |
| Operating Costs | Lower; standard components and longer maintenance intervals. | Higher; specialized parts and more frequent preventive maintenance. |
| Consumables | Columns last longer; higher solvent use increases waste and cost over time. | Columns are more expensive and shorter-lived but offset by reduced solvent consumption. |
| Solvent Consumption | Higher; contributes to greater long-term running costs and waste generation. | Lower; significantly reduces solvent purchase and disposal costs. |
| Overall Cost-Efficiency | Cost-effective for routine, validated applications. | Economically justified in high-throughput or development environments where speed and efficiency are priorities. |
Instrument Investment
UPLC systems require hardware capable of sustaining pressures above 1,000 bar. Pumps, seals, and tubing are built to tighter tolerances, which increases the acquisition cost by 30–50% compared to standard HPLC systems. Laboratories considering a full transition often factor in the expense of upgraded autosamplers, detector cells, and dedicated software licenses. HPLC, by contrast, is widely available and far more economical for labs that prioritize established methods over analytical speed.
Operating Costs and Consumables
Although UPLC instruments are more expensive, they offset part of that investment through lower solvent use. With flow rates typically a fraction of those used in HPLC, UPLC can reduce mobile-phase consumption by up to 70%. This not only cuts solvent expenses but also reduces waste disposal costs, a growing concern under sustainability-driven regulations.
However, the columns themselves are more costly and tend to have shorter lifespans due to higher operating pressure and sensitivity to fouling. HPLC columns, while less efficient, can run hundreds of injections with minimal degradation, keeping long-term consumable costs lower for routine testing.
Maintenance and Downtime
UPLC’s high-pressure operation leads to more frequent replacement of seals, frits, and pump heads. Preventive maintenance schedules must be followed more closely to avoid pressure instability or baseline drift. For multi-instrument QC labs, this can mean higher yearly maintenance costs and potential downtime for recalibration. HPLC systems are simpler to service, and many replacement parts are interchangeable across models, lowering both cost and turnaround time.
Productivity and Cost Efficiency
The main cost advantage of UPLC becomes evident in high-throughput environments. Shorter runtimes translate to higher sample throughput, freeing analysts and instruments for additional work. When amortized over time, the higher purchase price of UPLC can be offset by improved productivity and solvent savings. Conversely, in QC laboratories with fixed sample volumes and validated compendial methods, the cost advantage of UPLC is rarely realized, making HPLC the more economical long-term solution.
In implementation terms, HPLC remains cost-effective for validated, routine testing, while UPLC provides a higher return on investment in R&D and screening environments where speed and resolution directly influence project timelines and resource utilization.
Future Perspectives
As chromatographic technologies advance, both HPLC and UPLC continue to evolve toward faster analyses, higher resolution, and smarter automation. While the choice between them still depends on analytical purpose and regulatory context, new instrument designs and column materials are steadily narrowing the gap, redefining what laboratories can expect from routine testing and development workflows.
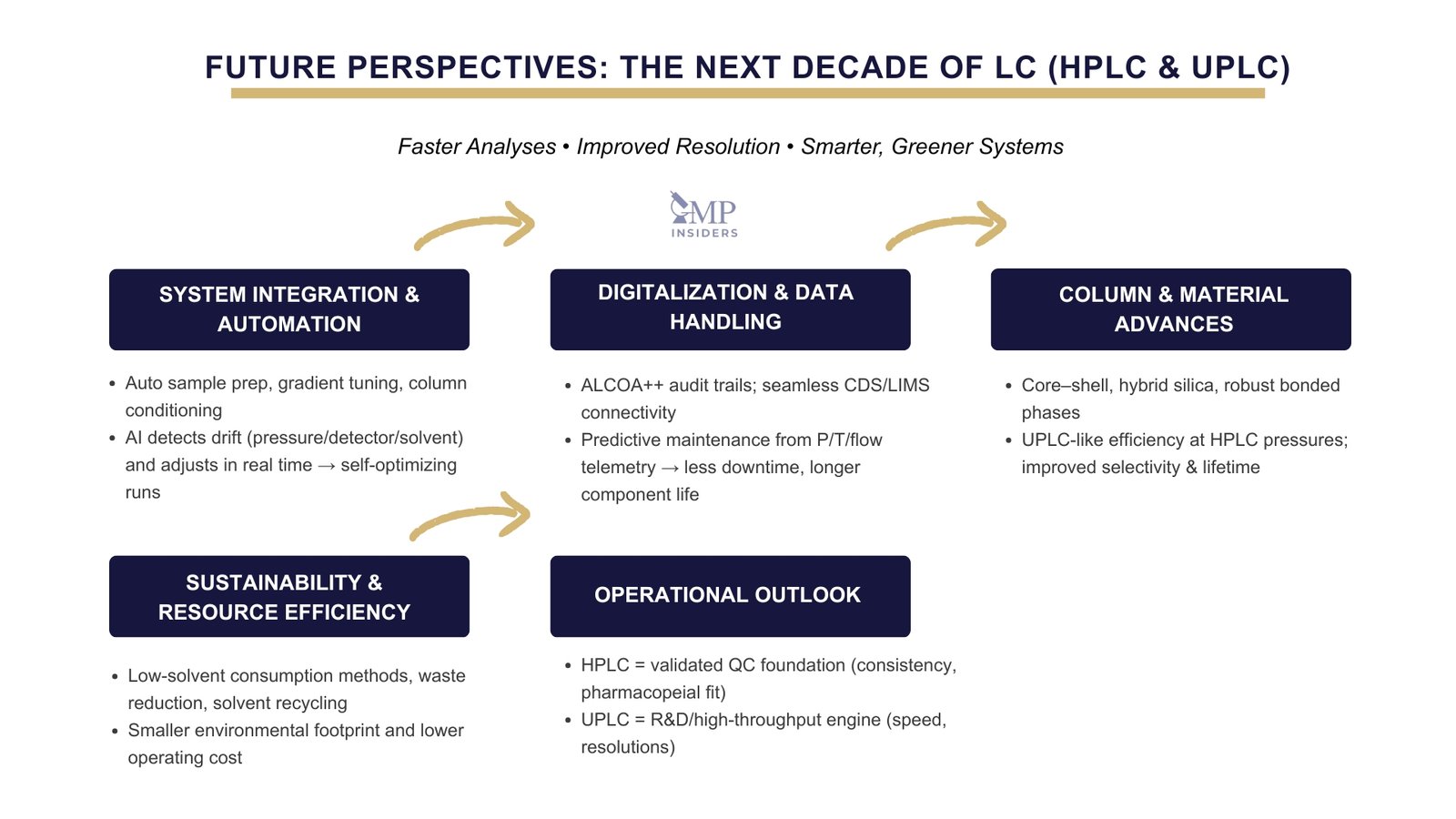
System Integration and Automation
Modern chromatographic systems are shifting from isolated instruments to fully integrated analytical platforms. The latest HPLC and UPLC designs combine automated sample preparation, gradient optimization, and column conditioning with built-in diagnostic intelligence. By integrating AI-based control algorithms, these systems can recognize early signs of pressure fluctuations, detector drift, or solvent instability and adjust parameters in real time. This evolution reduces manual oversight, shortens recovery time after deviations, and ensures stable performance across consecutive runs, a clear step toward self-optimizing laboratories.
Digitalization and Data Handling
With data integrity and traceability remaining central to GMP compliance, modern HPLC and UPLC systems now feature enhanced audit trails and seamless connectivity to LIMS or CDS platforms. Advanced analytics and AI-assisted monitoring are beginning to transform how laboratories manage instrument reliability, using real-time data on pressure, temperature, and flow stability to predict maintenance needs before performance drifts occur. This approach minimizes downtime, extends component lifespan, and replaces reactive part replacement with data-driven preventive action.
Related Article: Data Integrity in Peak Integration
Column and Material Advancements
Emerging column technologies, including core–shell particles, hybrid silica materials, and more stable bonded phases, are extending lifetimes and improving selectivity. These innovations deliver UPLC-level efficiency at moderate pressures, providing QC labs with practical, cost-controlled alternatives.
Sustainability and Resource Efficiency
The push for greener operations continues to shape chromatographic development. Both systems are adopting low-solvent designs, improved waste-reduction features, and recyclable mobile phases, aligning analytical performance with environmental responsibility.
Related Article: Toxic Solvents in Reversed Phase Chromatography
Operational Outlook
HPLC will remain the backbone for validated and pharmacopeial methods where consistency and regulatory familiarity are critical. UPLC will expand its role in R&D, impurity profiling, and high-throughput analysis. The convergence of design, automation, and sustainability will ensure both systems continue to complement each other in the modern pharmaceutical laboratory.
FAQ
How Can Laboratories Justify the Transition From HPLC to UPLC to Regulatory Inspectors During Audits?
Regulators expect a clear scientific rationale and documented demonstration of equivalence. The transition from HPLC to UPLC must be justified through comparative validation or partial revalidation, covering parameters such as accuracy, precision, linearity, range, specificity, and robustness.
A well-structured method equivalency protocol should define acceptance criteria for key performance metrics (e.g., resolution, retention factor, plate count, tailing factor) and include side-by-side chromatograms to illustrate consistency.
Many laboratories support this justification with a risk-based impact assessment:
Low risk if scaling affects only column geometry and flow rate, with no change in chemistry or detection principle.
Moderate to high risk if gradient behavior, dwell volume, or system pressure differ significantly.
In these cases, inspectors typically expect evidence of comparative system suitability results, updated method validation summary reports, and change-control documentation aligned with ICH Q2(R2), USP <621>, and the lab’s internal Analytical Method Lifecycle Management procedures.
What Challenges Typically Arise When Transferring Legacy HPLC Methods With Gradient Elution to UPLC Platforms?
Gradient-based methods are especially sensitive to dwell-volume mismatches, the extra volume between the point of gradient mixing and the column inlet. UPLC systems often have lower dwell volumes and faster gradient response times, which can shift retention and alter relative peak spacing or selectivity.
Other typical issues include:
Re-optimization of gradient slope: shorter column volumes require proportionally shorter gradient times to maintain slope equivalence.
Extra-column dispersion effects: sharper peaks from UPLC columns can be distorted by poorly optimized tubing or detector cell volumes.
Detector response and sampling rate: UPLC peaks are narrower; detectors must operate with faster data acquisition to maintain peak integrity.
Solvent-mixing differences: HPLC quaternary systems versus UPLC binary pumps can alter gradient composition profiles, impacting reproducibility.
Mitigation strategies involve gradient-time recalculation using volume-ratio formulas, dwell-volume correction, and fine tuning of re-equilibration times. Some laboratories also verify selectivity through peak purity and spectral overlays to confirm the method’s integrity post-transfer.
How Do Laboratories Determine When Solvent Savings From UPLC Offset the Higher Instrument and Column Costs?
The financial justification depends primarily on analytical throughput and solvent consumption patterns. UPLC systems typically use 60–80 % less mobile phase per run, and with faster cycle times, daily sample capacity can double or triple.
A cost–benefit analysis often includes:
Solvent cost reduction: acetonitrile or methanol usage decreases significantly, improving both cost efficiency and environmental sustainability.
Waste-disposal savings: reduced solvent waste lowers disposal costs and aligns with green-chemistry initiatives.
Labor and productivity gains: analysts spend less time per batch, and instrument availability improves for other tasks.
In high-throughput QC or stability testing labs, the investment can be recovered within 1–2 years. Conversely, in low-volume or release-testing environments, return on investment may take longer, making hybrid deployment (HPLC for legacy assays, UPLC for new methods) a balanced approach.
What Preventive Maintenance Practices Are Critical for Maintaining Consistent UPLC Performance Under High-Pressure Operation?
UPLC operates at pressures exceeding 15,000 psi, making preventive maintenance essential for reliability and data integrity.
Key practices include:
Regular replacement of pump seals, check valves, and piston heads to prevent pulsation and flow inconsistency.
Frequent inspection of inlet filters and frits to avoid backpressure increases caused by particulates.
Scheduled calibration of pressure transducers and leak tests to confirm system integrity.
Routine column care: flushing with strong solvents after each sequence prevents blockages and carryover.
Monitoring system metrics: pressure profiles, baseline noise, and flow-rate accuracy are used in predictive maintenance programs that identify early signs of wear before failures occur.
Many laboratories now integrate maintenance logs into their LIMS or CDS, ensuring traceability and readiness for regulatory review.
Are There Regulatory or Pharmacopeial Barriers Preventing the Use of UPLC for Compendial Assays Originally Developed for HPLC?
There are no explicit prohibitions in any major pharmacopeia against using UPLC in place of HPLC. However, when substituting an HPLC-listed method, laboratories must demonstrate that the UPLC method produces equivalent or better results within the framework of method verification or revalidation.
According to USP <621> Chromatography, adjustments in column length, internal diameter, flow rate, particle size, and gradient time are permissible provided that resolution, signal-to-noise ratio, and selectivity remain acceptable.
Before full implementation, laboratories should:
Document the scientific rationale for equivalence and any adjustments made.
Compare system suitability test results against compendial criteria.
Record the method change under the site’s change-control and validation SOPs.
In practice, regulatory agencies accept UPLC as a modernized continuation of HPLC, provided the transfer is supported by objective data and traceable documentation demonstrating analytical comparability.
Conclusion
The comparison between HPLC and UPLC is not a matter of superiority but of purpose. HPLC remains the most reliable choice for validated, compendial, and routine testing where method robustness and regulatory alignment are critical. UPLC, on the other hand, offers a clear advantage in environments that demand faster turnaround, higher resolution, and lower solvent consumption.
Both systems will continue to coexist, HPLC as the dependable standard of quality control, and UPLC as the performance-driven tool for development and innovation. With ongoing advancements in automation, predictive maintenance, and column design, laboratories can expect more efficient, data-driven chromatography that combines the strengths of both platforms to support consistent, compliant, and forward-looking analytical practice.















