Packaging plays a decisive role in the pharmaceutical industry. It is not only the final step before a medicine reaches the patient but also a critical component of the overall quality system. When designed and controlled according to GMP requirements, packaging protects products from contamination, maintains their stability, and ensures that every batch remains identifiable and traceable.
When managed under GDP principles, packaging extends its function into the supply chain, preserving product integrity during transport and storage while protecting against tampering, diversion, and temperature excursions. The types of pharmaceutical packaging are commonly divided into primary, secondary, and tertiary. Each category has its own purpose, risks, and regulatory expectations.
A clear understanding of these packaging layers, along with their material options and regulatory requirements, is essential for professionals in manufacturing, quality assurance, and distribution. It ensures that medicines are consistently delivered in a safe, effective, and compliant manner, adhering to international standards.
Classification of Pharmaceutical Packaging
Pharmaceutical packaging can be grouped into three main categories: primary, secondary, and tertiary. Each serves a distinct role in protecting the product and maintaining compliance with GMP during manufacturing and GDP during distribution. Together, they form a layered system that safeguards the medicine from production to patient use.
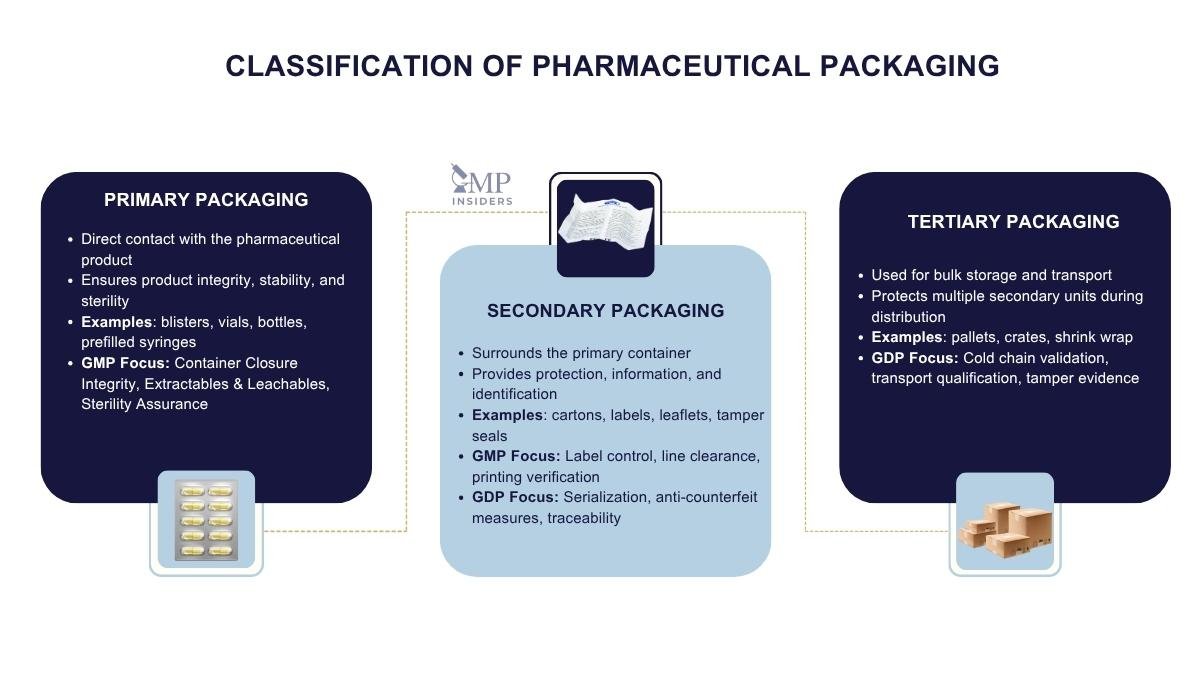
Primary Packaging
Primary packaging comes into direct contact with the pharmaceutical product and is therefore the most critical component in ensuring the product’s safety and stability. Its design must prevent any interaction with the drug while maintaining the product’s integrity throughout its shelf life.
Examples include:
- Blister packs for tablets and capsules
- Glass and plastic bottles for solids and liquids
- Ampoules, vials, and prefilled syringes for injectables
- Sachets and strips for powders or oral formulations
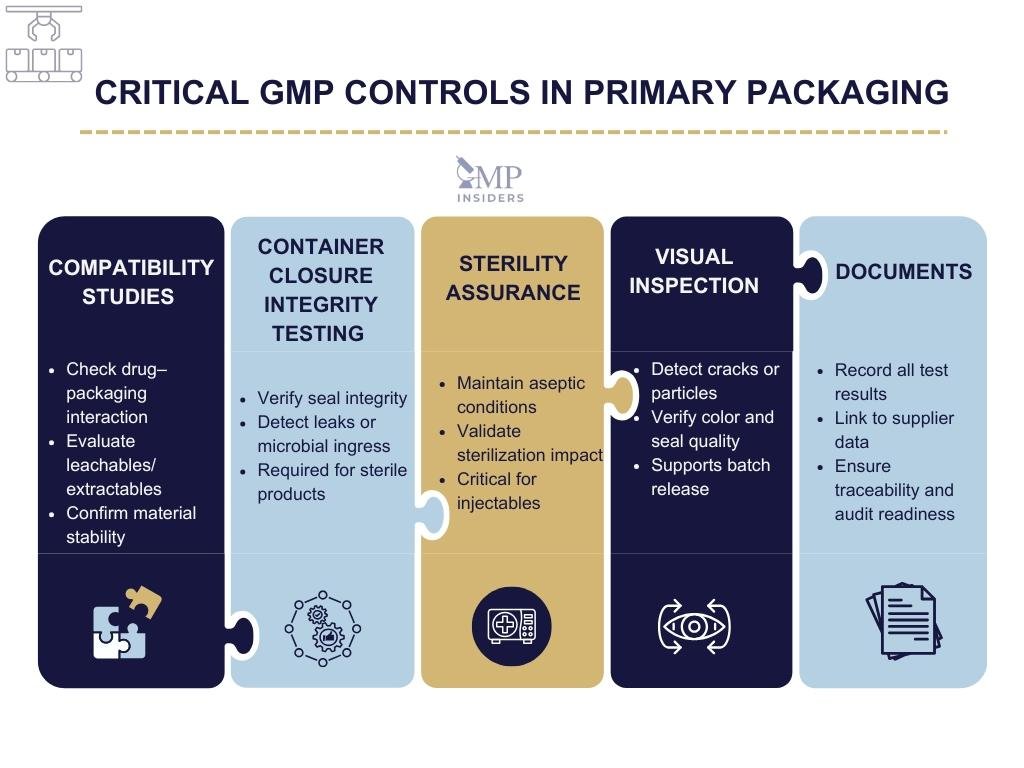
GMP focus areas:
- Container closure integrity testing (CCIT) to ensure no leaks or microbial ingress
- Compatibility studies to avoid leachables, extractables, or sorption of the active ingredient
- Sterility assurance for parenteral products
- Control of particulate matter and breakage risks
Risks to manage:
- Glass delamination in vials
- Migration of plasticizers or additives into the product
- Seal failures in blister packs or ampoules
- Moisture or oxygen permeation affecting product stability
Secondary Packaging
Secondary packaging surrounds the primary container, providing additional layers of protection and information. Although it does not directly contact the drug, it is essential for compliance and communication.
| Component | Function | Regulatory Focus | Common Issue | Preventive Measure |
|---|---|---|---|---|
| Label | Identification | FDA 21 CFR 211.122 | Mix-ups | Line clearance procedures |
| Leaflet | Patient information | EU Directive 2001/83/EC | Missing updates | SOP for document control |
| Carton | Physical protection | GMP Annex 9 | Damage | Packaging material qualification |
Examples include:
- Folding cartons and boxes
- Labels and leaflets with instructions for use
- Tamper-evident seals and overwraps
Key functions:
- Regulatory compliance: correct labeling with product information, batch numbers, expiry dates, and safety warnings
- GDP relevance: serialization, barcoding, and anti-counterfeit features for supply chain integrity
- Patient safety: clear instructions for storage, handling, and administration
- Branding and usability: user-friendly design to support adherence and avoid medication errors
Risks to manage:
- Label mix-ups during packaging operations
- Incorrect or unreadable printing leading to medication errors
- Counterfeit products entering the supply chain without adequate serialization
SEE ALSO: Primary vs Secondary Packaging in Pharmaceutical Industry
Tertiary Packaging
Tertiary packaging is designed for bulk storage and transport. It safeguards multiple units of secondary packaging and ensures medicines are delivered intact through complex distribution networks.
| Packaging Type | Use | Key GDP Controls | Common Risks | Mitigation |
|---|---|---|---|---|
| Pallets | Bulk transport and storage of product cartons | Load validation, pallet hygiene, traceability via barcodes or RFID | Crushing, contamination, loss of traceability | Standard stacking height, clean heat-treated pallets, validated wrapping |
| Insulated Boxes / Thermal Containers | Cold chain distribution of temperature-sensitive products | Packaging qualification, data logger monitoring, transport validation | Temperature excursions, logger failure | Validated shippers, pre-conditioned coolants, staff training |
| Shipping Cartons | Protection of secondary packs during storage and transit | Carton integrity, tamper-evident sealing, labeling for orientation | Damage, label loss, missing seals | Certified cartons, humidity control, label verification |
| Crates / Rigid Containers | Heavy or fragile product transport | Load validation, tamper-evident closure, inspection for integrity | Deformation, broken seals | Routine inspection, numbered seals, immediate replacement |
| Shrink Wrap / Stretch Film | Load stability and tamper evidence | Film quality check, wrapping validation, visual inspection | Tearing, poor containment, undetected tampering | Operator training, film integrity checks, dispatch inspection |
Examples include:
- Corrugated shipping cartons
- Pallets and crates
- Shrink wrap or stretch film
Key functions:
- Protection in transit: prevents mechanical damage, crushing, or environmental exposure
- GDP compliance: supports validated cold chain transport and protects products from temperature excursions
- Traceability: integration with track-and-trace systems through pallet-level barcoding or RFID tags
- Tamper resistance: visible signs if a shipment is compromised
Risks to manage:
- Loss of temperature control during long-distance shipping
- Physical damage caused by improper palletization
- Diversion or theft during distribution if seals are weak or traceability is missing
Materials Used in Pharmaceutical Packaging
Selecting the right material is one of the most critical steps in pharmaceutical packaging. The choice must balance product protection, manufacturability, regulatory compliance, and patient usability. Each material comes with its own strengths, limitations, and GMP testing requirements.

Glass
Glass remains one of the most widely used materials for pharmaceutical packaging, especially for injectables.
Types of glass:
- Type I Borosilicate – chemically resistant, suitable for sensitive injectables
- Type II Treated Soda-lime – surface treated for moderate chemical resistance
- Type III Soda-lime – limited chemical resistance, used for dry formulations
Advantages:
- Excellent barrier against moisture, oxygen, and other gases
- Chemically inert when properly selected
- Heat-resistant and compatible with sterilization
Risks to manage:
- Delamination: glass flakes that may contaminate injectables
- Breakage: fragile nature increases risk during handling and transport
- Particulate shedding if vials are not processed under controlled conditions
GMP controls:
- Container closure integrity testing
- Visual inspection for cracks and defects
- Supplier qualification and lot testing for delamination risks
Plastics
Plastic materials are increasingly used due to their flexibility, low weight, and cost-effectiveness. Common types include:
- HDPE (High-density polyethylene) – bottles for tablets and liquids
- LDPE (Low-density polyethylene) – squeezable containers
- PET (Polyethylene terephthalate) – excellent clarity, used for syrups and liquids
- PP (Polypropylene) – blister packs, closures, and syringes
- PVC (Polyvinyl chloride) – widely used in blister packs but under scrutiny due to sustainability concerns
Advantages:
- Lightweight and resistant to breakage
- Easier to mold into complex designs
- Lower transport costs compared to glass
Risks to manage:
- Permeability: some plastics allow oxygen, CO₂, or moisture transfer
- Leachables and extractables: risk of migration into drug products
- Environmental concerns: non-recyclable plastics under regulatory pressure
GMP controls:
- Comprehensive extractables and leachables (E&L) studies
- Compatibility studies with formulation solvents and excipients
- Controlled storage to prevent material degradation
Metals and Foils
Aluminum is the most commonly used metal in pharmaceutical packaging, primarily for closures and backing blister packs.
Applications:
- Blister pack lidding foils
- Aluminum tubes for ointments and creams
- Closures and caps for vials and bottles
Advantages:
- Excellent barrier against light, moisture, and gases
- Provides tamper-evident sealing
- Stable at a wide range of temperatures
Risks to manage:
- Potential for corrosion if improperly coated
- Interaction with highly reactive formulations
- Risk of pinholes or defects in foils compromising barrier properties
GMP controls:
- Incoming inspection for foil integrity
- Coating integrity verification
- Regular qualification of sealing equipment
Paper and Laminates
Paper and composite laminates are mainly used in secondary and tertiary packaging.
Applications:
- Folding cartons, labels, and leaflets
- Outer shipping boxes and crates
- Laminated paper foils for flexible pouches
Advantages:
- Cost-effective and lightweight
- Easy to print with essential information
- Increasingly recyclable and eco-friendly
Risks to manage:
- Poor barrier properties, unsuitable for direct drug contact
- Susceptible to moisture and mechanical damage
- Counterfeit risks if labeling and serialization are not robust
GMP controls:
- Verification of printed data accuracy and legibility
- Controls for mix-ups during packaging operations
- Supplier audits to confirm compliance with pharmaceutical-grade paper standards
Combination and Advanced Materials
Modern pharmaceutical packaging often uses multi-layer laminates or hybrid materials to achieve superior protection.
Examples:
- Plastic-aluminum laminates for high-barrier blister packs
- Coated glass vials to reduce delamination risks
- Child-resistant and senior-friendly packaging systems
Trends:
- Increasing focus on recyclable and biodegradable materials
- Smart materials integrated with track-and-trace features
- Development of barrier films with improved stability profiles
Emerging Trends in Pharmaceutical Packaging
Pharmaceutical packaging is evolving rapidly to meet new regulatory, technological, and environmental demands. While its core function remains product protection, modern packaging is increasingly expected to support patient safety, supply chain transparency, and sustainability.
| Trend | Technology / Material | Purpose | Regulatory Reference |
|---|---|---|---|
| Smart Packaging | RFID / NFC | Traceability & authentication | EU FMD, DSCSA |
| Child-Resistant Closures | CRC systems | Safety | ISO 8317 |
| Sustainable Packaging | Biodegradable polymers | Eco compliance | EMA Reflection Paper |
| Barrier Coatings | SiO₂-coated vials | Stability | USP <1207> CCIT |
| Serialization | 2D barcodes | Anti-counterfeit | EU FMD, 21 CFR 232 |
Smart Packaging
Advances in digital technology are driving the development of intelligent packaging solutions.
- Serialization and barcoding: mandatory in many regions to support traceability and combat counterfeiting.
- RFID and NFC tags: enable real-time tracking of product movement and authentication at any point in the supply chain.
- Digital adherence tools: packaging that records when a dose is removed, supporting patient compliance in clinical trials or chronic therapies.
Regulatory impact: authorities encourage digital solutions for product security, particularly under EU FMD and US DSCSA.
Tamper-Evident and Child-Resistant Features
Patient safety is reinforced by packaging that provides visible evidence of interference and reduces risks of accidental exposure.
- Tamper-evident seals: shrink bands, tear strips, or breakable caps that show visible damage when opened.
- Child-resistant closures (CRC): widely required for oral solid dose forms to prevent accidental ingestion.
- Senior-friendly designs: balancing safety with ease of use for elderly patients, reducing medication errors.
Key expectation: these features must be tested under realistic conditions and validated before use in production.
Sustainable and Eco-Friendly Packaging
Environmental considerations are becoming a central part of pharmaceutical packaging strategies. Regulators, healthcare providers, and patients expect reduced environmental impact without compromising safety.
- Recyclable materials: increased use of PET and coated glass that allow easier recycling.
- Biodegradable plastics: being explored as alternatives to PVC blisters.
- Reduction of packaging volume: minimizing carton size and using lighter tertiary materials.
- Eco-labeling: highlighting compliance with environmental standards.
Industry challenge: ensuring that greener materials still meet GMP expectations for barrier protection, stability, and sterility.
Advanced Barrier Materials
Pharmaceutical products, particularly biologics and sensitive formulations, require packaging with enhanced protection against oxygen, moisture, and light.
- Multi-layer laminates: combining plastic, aluminum, and special coatings for high-barrier properties.
- Coated glass vials: reducing delamination and particle risk.
- High-performance films: preventing degradation in moisture- or oxygen-sensitive drugs.
Regulatory focus: all barrier materials must undergo full extractables and leachables studies.
Track-and-Trace Systems
Global regulations have made serialization and track-and-trace a fundamental requirement.
- EU Falsified Medicines Directive (FMD): serialization codes and anti-tamper devices are mandatory.
- US DSCSA: stepwise implementation of electronic, interoperable systems to trace products through the supply chain.
- Integration with logistics systems: enabling real-time visibility of product location, condition, and chain of custody.
Regulatory Requirements
Pharmaceutical packaging is tightly regulated because it has a direct impact on product quality, patient safety, and supply chain integrity. Both GMP and GDP guidelines establish clear expectations for selecting, testing, and controlling packaging materials throughout the product lifecycle.
GMP Requirements
Under Good Manufacturing Practice, packaging is treated as an extension of the drug product. The quality of packaging components is considered critical to maintaining the safety, identity, strength, purity, and quality of the medicine.
Key regulatory references:
- EU GMP: Annex 1 (sterile packaging requirements), Annex 9 (packaging materials), Chapter 5 (production), Chapter 6 (quality control)
- US FDA 21 CFR Part 211: Subpart G (Packaging and Labeling Control), Subpart E (Control of Components and Drug Product Containers and Closures)
- ICH Q8, Q9, Q10: framework for product design, risk management, and pharmaceutical quality system
Main expectations:
- Material qualification: suppliers of packaging components must be qualified and audited
- Testing requirements: identity, quality, compatibility, and stability testing of materials
- Container Closure Integrity: demonstrated through validated CCIT methods
- Prevention of mix-ups: controls during labeling and packaging operations
- Documentation: batch records must include packaging line checks, reconciliation of labels, and inspection results
GDP Requirements
Good Distribution Practice expands packaging responsibilities beyond the manufacturing site into the logistics and supply chain network. Packaging must ensure the product remains safe and effective throughout storage, handling, and transport.
Key regulatory references:
- EU GDP Guidelines (2013/C 343/01): Sections on storage, transport, and protection against falsification
- WHO Annex 9: global reference on packaging for pharmaceutical products
- National authorities: local requirements for cold chain transport and serialization
Main expectations:
- Protection in transit: validated packaging systems that maintain product stability (e.g., temperature-controlled containers for biologics)
- Tamper-evident features: visible evidence of any unauthorized access
- Serialization and traceability: mandatory under the EU Falsified Medicines Directive and DSCSA in the United States
- Risk-based transport validation: packaging qualified for expected stress factors such as temperature fluctuations, vibration, or humidity
- Documentation and monitoring: distribution records, temperature logs, and deviation handling
FAQ
What Role Does Packaging Play in Protecting Against Counterfeit Medicines?
Pharmaceutical packaging is the first line of defense against counterfeit products. Features like holograms, color-shifting inks, QR codes, and serialization codes make it harder for counterfeiters to reproduce authentic packaging.
Regulators, such as the EU through the Falsified Medicines Directive, mandate serialization systems to trace products across the supply chain. By embedding advanced security features, packaging protects both patients and manufacturers from counterfeit-related risks.
Why Are Stability Studies Important for Packaging Systems?
Stability studies verify that the packaging maintains the drug’s safety and efficacy over its intended shelf life. These studies subject packaging to accelerated and long-term conditions such as temperature, humidity, and light exposure. Results determine appropriate storage conditions and expiry dates. Without stability data, packaging systems cannot be fully qualified for GMP use.
What Is the Importance of Packaging Line Clearance?
Line clearance prevents mix-ups and cross-contamination between batches or products. Before a new batch is packaged, all leftover materials, labels, and product residues must be removed and documented.
Automated sensors, barcode readers, and manual double checks are often used to verify that the line is clear. Failure to perform proper line clearance can lead to regulatory findings and product recalls.
Why Are Extractables and Leachables Studies Critical for Packaging Development?
Extractable and Leachables studies identify and quantify substances that may migrate from packaging materials into the drug product. Extractables are potential compounds identified under exaggerated conditions, whereas leachables are those that actually migrate under normal storage conditions.
Such impurities can alter product safety, stability, and efficacy. Regulatory authorities expect comprehensive E&L evaluations before packaging can be approved.
What Role Does Packaging Play in Pharmacovigilance?
Packaging contributes to pharmacovigilance by ensuring the accurate identification of products in the event of an adverse event report. Batch numbers, serialization codes, and expiry dates printed on packaging allow traceability during investigations. Incorrect or missing data could delay root cause analysis and risk mitigation. Thus, reliable packaging supports the broader pharmacovigilance framework.
Final Thoughts
Pharmaceutical packaging is more than a container for medicines. It is a critical element of GMP and GDP compliance that protects product quality, ensures patient safety, and maintains integrity across the global supply chain. From the primary container in direct contact with the drug to the tertiary systems used for bulk transport, every layer of packaging has defined responsibilities, regulatory expectations, and risks that must be carefully managed.
Packaging today is expected to go beyond product protection. It plays a role in serialization, track and trace, tamper evidence, and the fight against falsification. It also responds to increasing expectations for sustainability, as companies adopt recyclable, biodegradable, and eco-efficient materials without compromising safety and quality.
For professionals in manufacturing, quality assurance, and supply chain, packaging must be viewed as an integral part of the pharmaceutical quality system. It is not simply a container but a safeguard of patient trust, compliance, and product integrity. Companies that invest in robust packaging systems and embrace innovation will be better equipped to meet future regulatory challenges and global distribution demands.















