The validation steps—Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ)—are vital for confirming that equipment and systems are installed and functioning correctly and consistently.
This article details the importance, procedures, and regulatory standards associated with IQ, OQ, and PQ, highlighting their roles in maintaining high-quality manufacturing practices.
What is Installation Qualification (IQ)?
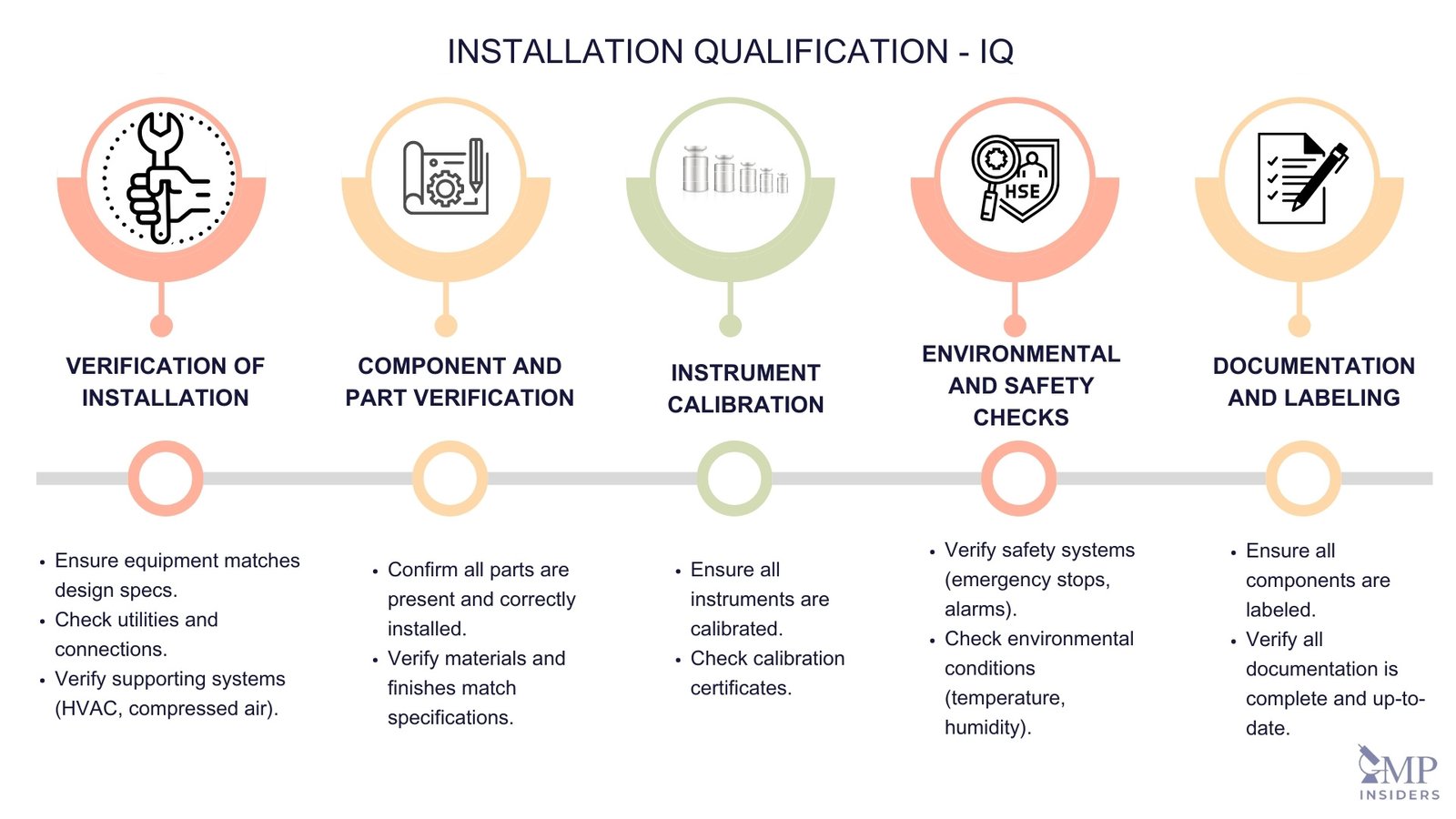
Installation Qualification (IQ) - The documented verification that the facilities, systems, and equipment, as installed or modified, comply with the approved design and the manufacturer's recommendations.
Before IQ, a Design Qualification (DQ) should be performed to verify that the proposed design meets operational needs.
RELATED: Design Review and Design Qualification in the Pharmaceutical Industry
Key Objectives of IQ:
- Verify Installation: Confirm that the equipment or system has been installed according to the design specifications and manufacturer’s recommendations.
- Ensure Completeness: Ensure all required components, documentation, and utilities are present and correctly installed.
- Baseline for Ongoing Validation: Establish a documented baseline for the equipment’s condition and configuration, which is essential for future qualification and validation efforts.
Key Elements of IQ
The key elements of IQ include:
Verification of Installation
- Equipment Specifications: Check that the equipment matches the specified design and that all components are installed as per the manufacturer’s guidelines.
- Utilities and Connections: Verify that all necessary utilities (electricity, water, air, etc.) and connections (plumbing, wiring, etc.) are correctly installed and functional.
- Supporting Systems: Ensure that any supporting systems (e.g., HVAC, compressed air, vacuum systems) are properly installed and operational.
Component and Part Verification
- Parts List: Verify that all parts and components listed in the design specifications and purchase order are present and correctly installed.
- Material and Finish: Confirm that the materials used in the construction of the equipment match the specifications, including surface finishes and coatings.
Instrument Calibration
- Calibration Status: Ensure that all measuring and monitoring instruments associated with the equipment are calibrated and that calibration certificates are available.
- Reference Standards: Use traceable calibration standards to verify the accuracy of instruments.
Environmental and Safety Checks
- Safety Systems: Verify that all safety systems and features (e.g., emergency stops, alarms, interlocks) are installed and functional.
- Environmental Conditions: Check that the installation environment meets the required conditions, such as temperature, humidity, and cleanliness.
Documentation and Labeling
- Identification Labels: Ensure all components and equipment are correctly labeled with identification tags and serial numbers.
- Documentation Review: Verify that all relevant documentation (e.g., user manuals, maintenance manuals, engineering drawings) is available and up-to-date.
IQ Documentation Requirements
Proper documentation is a cornerstone of the IQ process, providing a detailed record that the equipment or system was installed correctly. Key documentation requirements include:
1. Installation Qualification Protocol
- Protocol Document: A detailed, pre-approved protocol outlining the scope, objectives, and procedures for the IQ process.
- Acceptance Criteria: Clearly defined acceptance criteria for each aspect of the installation that will be verified.
2. Equipment Specifications and Manuals
- Design Specifications: Documentation of the design specifications, including technical drawings, parts lists, and manufacturer’s instructions.
- User and Maintenance Manuals: Manufacturer’s manuals for operation, maintenance, and troubleshooting of the equipment.
3. Calibration Certificates
- Certificates: Calibration certificates for all measuring instruments, showing traceability to national or international standards.
- Calibration Records: Records of the calibration process, including dates, results, and personnel involved.
4. Installation Checklists
- Component Checklists: Detailed checklists for verifying the presence and correct installation of all components and parts.
- Utility Checklists: Checklists to confirm that all necessary utilities and connections are in place and functional.
5. Test and Verification Records
- Verification Results: Records of all tests and verifications performed during the IQ process, including results and any deviations noted.
- Corrective Actions: Documentation of any corrective actions taken to address deviations or non-conformances found during installation.
6. Final Report
- Summary Report: A comprehensive report summarizing the IQ process, including the protocol, test results, deviations, corrective actions, and a conclusion.
- Approval Signatures: Signatures of the personnel who conducted the IQ, as well as approvals from QA and other relevant departments.
What is Operational Qualification (OQ)?
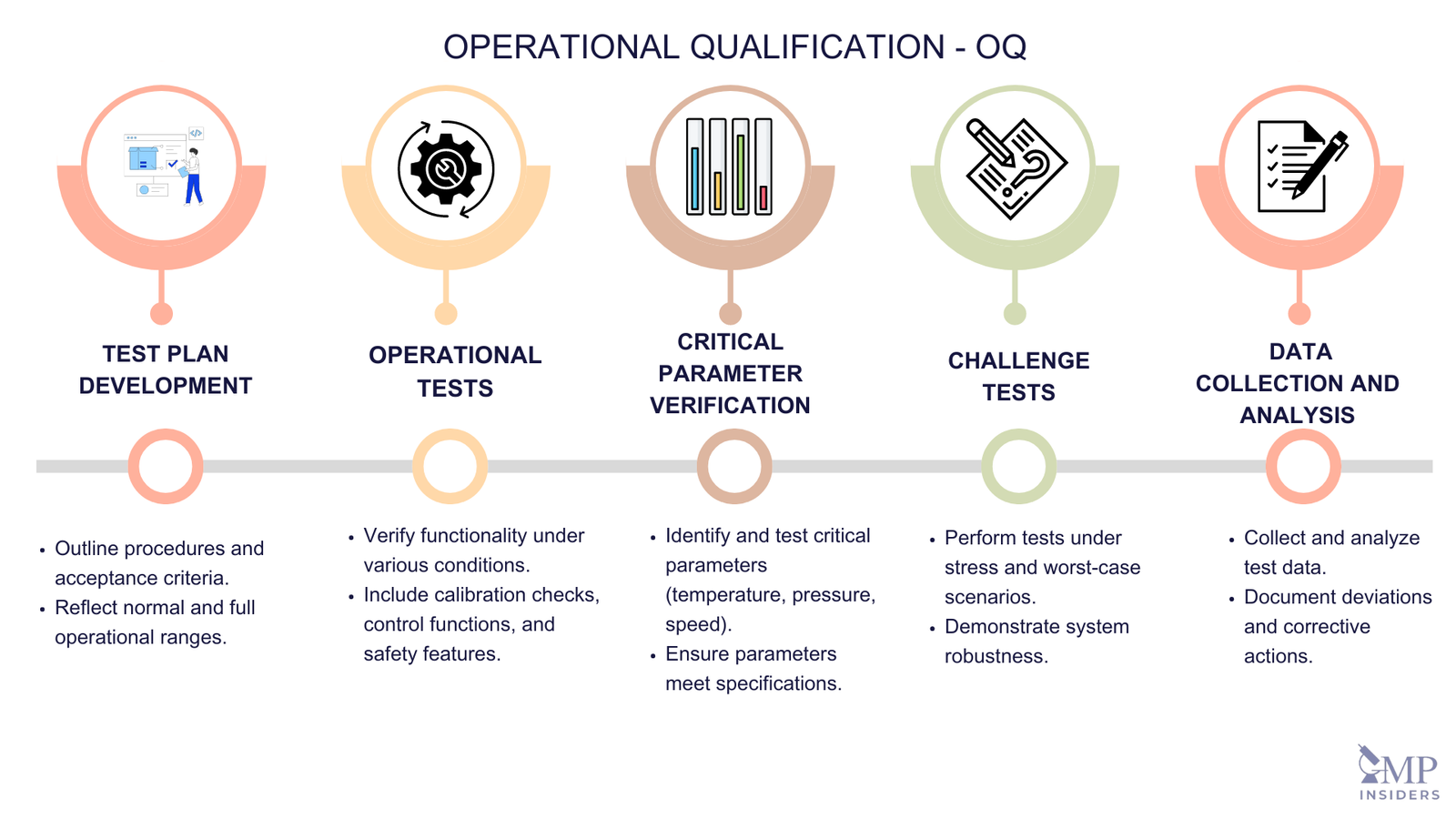
Operational Qualification (OQ) – The documented verification that the facilities, systems, and equipment, as installed or modified, perform as intended throughout the anticipated operating ranges. (Annex 15)
OQ typically follows Installation Qualification (IQ), but for complex equipment, it can be performed as a combined Installation/Operation Qualification (IOQ).
Key Elements of OQ
The key elements of OQ include:
Test Plan Development
- Establishing a Detailed Test Plan: Outlining the procedures, acceptance criteria, and test parameters. This plan should reflect the normal operating conditions and the full range of operational settings.
Operational Tests
Conducting Tests: Verify the system’s functionality under various conditions, including normal and stress conditions.
Tests Typically Cover:
- Calibration checks
- Control functions (e.g., alarms, interlocks)
- Operational ranges
- Software and automation systems
- Safety features
Critical Parameter Verification
- Identifying and Testing Critical Operational Parameters such as temperature, pressure, speed, time, and other relevant parameters that could impact product quality or process performance.
Challenge Tests
- Performing Challenge Tests: To demonstrate that the system can handle variations in operational conditions and still maintain performance. This may include testing limits or worst-case scenarios.
Data Collection and Analysis
- Collecting and Analyzing Data: From the tests to confirm that the equipment or system meets the predefined criteria. This includes statistical analysis and comparison with expected outcomes.
Deviation Management
- Documenting and Addressing Deviations: From expected results. This involves root cause analysis, corrective actions, and re-testing as necessary.
OQ Documentation Requirements
The key documentation requirements for OQ include:
1. OQ Protocol
- A comprehensive document outlining the scope, objectives, responsibilities, procedures, test methods, acceptance criteria, and references for the OQ phase. The protocol must be approved before execution.
2. Test Plans and Procedures
- Detailed test plans and standard operating procedures (SOPs) for conducting each test. These documents ensure consistency and reproducibility of the OQ process.
3. Test Records and Data
- Complete and accurate records of all tests performed, including raw data, test results, and observations. This documentation serves as evidence that the OQ was conducted as per the protocol.
4. Calibration Certificates
- Certificates or records of calibration for all measurement and test equipment used during OQ. This ensures that the equipment used for testing is accurate and reliable.
5. Deviation Reports
- Reports detailing any deviations from the protocol, including the nature of the deviation, investigation results, impact assessment, corrective actions taken, and re-testing results.
6. Summary Report
- A final report summarizing the OQ activities, results, and conclusions. This report should state whether the equipment or system has met the acceptance criteria and is suitable for proceeding to the next validation phase (Performance Qualification).
What is Performance Qualification (PQ)?
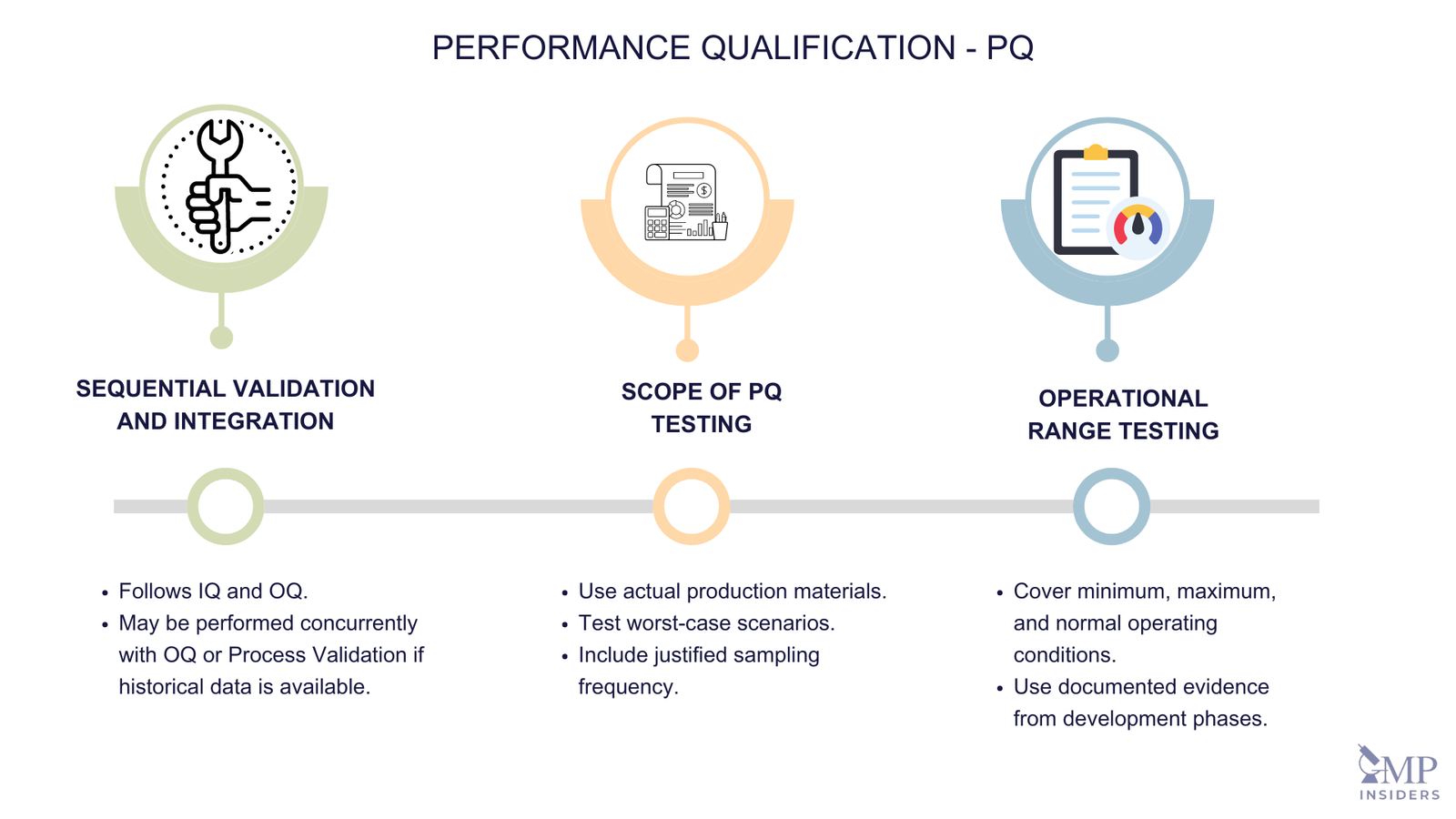
Performance Qualification (PQ) – The documented verification that systems and equipment can perform effectively and reproducibly based on the approved process method and product specification. (Annex 15)
PQ ensures the equipment’s reliability and effectiveness in producing consistent results over time.
Key Elements of PQ
The key elements of PQ include:
Sequential Validation and Integration
- Sequential Approach: PQ usually follows the successful completion of IQ and OQ to ensure that the equipment and systems are installed correctly (IQ) and operate according to specifications (OQ).
- Concurrent Validation: In certain scenarios, it may be appropriate to perform PQ in conjunction with OQ or Process Validation. This approach can be practical for processes with extensive historical data or well-established development studies.
Scope of PQ Testing
PQ should include, but is not limited to, the following aspects:
a. Testing with Production Materials
- Use of Actual Production Materials: PQ tests should be conducted using actual production materials to simulate real manufacturing conditions.
- Qualified Substitutes or Simulated Products: When actual production materials are not feasible, qualified substitutes or simulated products proven to have equivalent behavior can be used.
- Worst Case Scenarios: Testing should include worst-case batch sizes to challenge the system under the most demanding conditions expected in routine production.
b. Frequency of Sampling
- Justified Sampling Frequency: The frequency of sampling during PQ must be justified and based on the criticality of the process parameters. It ensures adequate process control and provides sufficient data to demonstrate consistent performance.
3. Operational Range Testing
- Covering Operating Range: PQ tests should encompass the entire operating range of the intended process. This includes testing at the minimum, maximum, and normal operating conditions to ensure the process is robust and capable of producing quality products under varying conditions.
- Documented Evidence: If there is documented evidence from the development phases that confirms the operational ranges, this can be used to support the PQ process. This evidence may include data from earlier development studies or pilot-scale runs.
PQ Documentation Requirements
The key documentation for PQ include:
1. PQ Protocol
- Scope and Objectives: Overview of the PQ process, including objectives and scope.
- Responsibilities: Clear definition of roles and responsibilities.
- Procedures and Test Methods: Detailed description of procedures and methods to be used.
- Acceptance Criteria: Specific criteria that must be met for PQ to be considered successful.
2. Test Plans and Procedures
- Detailed Test Plans: Comprehensive plans outlining each test to be conducted.
- Standard Operating Procedures (SOPs): SOPs to ensure consistency and reproducibility.
3. Test Records and Data
- Raw Data: Complete records of all tests performed, including raw data, test results, and observations.
- Data Analysis: Analysis of the test data, including statistical evaluations.
4. Calibration Certificates
- Calibration Records: Certificates or records of calibration for all measurement and test equipment used during PQ, ensuring accuracy and reliability.
5. Deviation Reports
- Documentation: Detailed reports of any deviations from the protocol.
- Investigation and Actions: Root cause analysis, impact assessment, corrective actions taken, and re-testing results.
6. Summary Report
- Summary of Activities: Overview of the PQ activities, results, and conclusions.
- Conclusions: Statement on whether the equipment/system meets the acceptance criteria and is suitable for production use.
Steps in the Validation Process
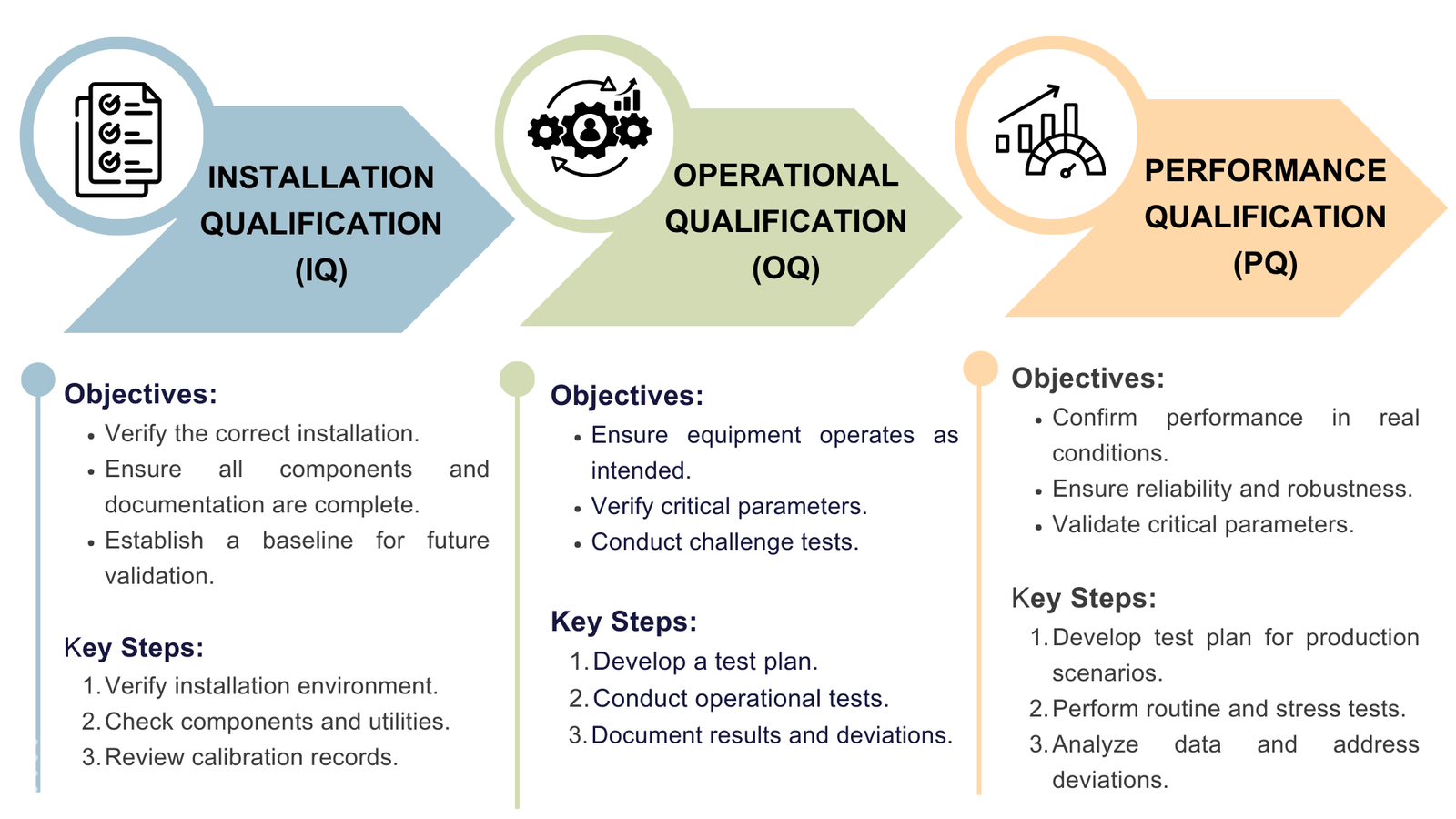
The key steps when performing IQ, OQ, and PQ include:
Step 1: Write and Develop the Protocol
When developing IQ, OQ or PQ Protocol here are the main factors to consider and include:
1.1 Define the Scope and Objectives:
- Scope: Clearly outline what the protocol will cover. This includes specifying the equipment, system, or process being validated.
- Objectives: Define the goals of the validation effort, such as ensuring compliance with regulatory requirements, verifying performance specifications, and establishing documented evidence of system suitability.
1.2 Assemble a Validation Team:
- Include representatives from relevant departments such as Quality Assurance (QA), Production, Engineering, and Validation.
- Define the roles and responsibilities of each team member.
1.3 Develop the Validation Plan:
- Plan Outline: Provide a high-level overview of the entire validation process.
- Resources: List the resources required, including personnel, equipment, and materials.
- Schedule: Develop a timeline for the validation activities.
1.4 Draft the Protocol Document:
- Protocol Title and Identification: Assign a unique title and identification number to the protocol.
- Introduction and Background: Describe the purpose of the protocol and provide background information.
- Scope: Reiterate the scope of the validation.
- Responsibilities: Detail the responsibilities of each team member.
- Acceptance Criteria: Define clear and measurable acceptance criteria for each validation activity.
- Procedure: Provide detailed step-by-step instructions for conducting the validation.
- Documentation: Specify the types of records that need to be kept and how they should be documented.
- Change Control: Outline the process for handling deviations and changes during validation.
1.5 Review and Approval:
- Circulate the draft protocol among key stakeholders for review.
- Address any comments or concerns raised during the review.
- Obtain formal approval from relevant authorities, such as QA and regulatory affairs.
Step 2: Execute an IQ, OQ, or PQ Protocol
2.1 Preparation for Execution:
- Training: Ensure that all personnel involved in the validation have received proper training on the protocol and the equipment or process being validated.
- Equipment and Materials: Confirm that all necessary equipment and materials are available and in good working condition.
- Documentation: Prepare all forms, checklists, and data sheets required for recording validation results.
2.2 Conduct Installation Qualification (IQ):
- Purpose: Verify that the equipment or system is installed correctly and according to the manufacturer’s specifications.
- Procedure:
- Verify the installation site environment.
- Check and document the condition and presence of all components.
- Confirm that the equipment is installed according to the design specifications.
- Verify that all utilities (e.g., electrical, water, air) are correctly connected.
- Review calibration and maintenance records.
- Documentation: Record all findings, including any discrepancies and actions taken to resolve them.
2.3 Conduct Operational Qualification (OQ):
- Purpose: Ensure that the equipment or system operates as intended throughout all anticipated operating ranges.
- Procedure:
- Develop and execute test cases that cover all functional aspects of the equipment or system.
- Verify that operational controls, alarms, and interlocks function correctly.
- Perform repeated tests to demonstrate consistent performance.
- Document test results, deviations, and corrective actions.
- Acceptance Criteria: Results must meet predefined acceptance criteria to confirm that the equipment or system operates correctly.
2.4 Conduct Performance Qualification (PQ):
- Purpose: Demonstrate that the equipment or system performs consistently and effectively under real-world conditions.
- Procedure:
- Develop test cases that simulate normal operating conditions.
- Include tests for all critical parameters and performance characteristics.
- Monitor the system over an extended period to ensure consistent performance.
- Document all results, including any deviations and corrective actions.
- Documentation: Compile all data and evidence showing that the system performs as expected in the actual production environment.
2.5 Review and Report:
- Review: Conduct a thorough review of all documentation and results from IQ, OQ, and PQ activities.
- Report: Prepare a comprehensive validation report that summarizes the findings, including any deviations and how they were addressed.
- Approval: Obtain final approval of the validation report from QA and other relevant stakeholders.
2.6 Continuous Monitoring and Revalidation:
- Establish a program for ongoing monitoring of the equipment or system to ensure continued compliance.
- Define criteria and a schedule for periodic revalidation based on changes to the system or process or as part of routine quality assurance.
By following these steps meticulously, you can ensure that the validation process is thorough, systematic, and compliant with GMP guidelines.
Regulatory Requirements for IQ, OQ, and PQ
The regulatory requirements for Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) in Good Manufacturing Practice (GMP) are outlined by various international regulatory bodies such as the FDA (Food and Drug Administration) and EMA (European Medicines Agency). These qualifications are essential components of the validation process for manufacturing equipment, systems, and processes in the pharmaceutical industry. Below is a detailed overview of the regulatory requirements:
FDA (Food and Drug Administration)
- Code of Federal Regulations (CFR): The FDA’s requirements for IQ, OQ, and PQ are detailed in 21 CFR Part 211 (Current Good Manufacturing Practice for Finished Pharmaceuticals) and 21 CFR Part 820 (Quality System Regulation for Medical Devices).
- 21 CFR Part 211:
- §211.68: Automated, mechanical, and electronic equipment should be routinely calibrated, inspected, or checked according to a written program designed to assure proper performance.
- §211.100: Requires validation of all processes that could affect the quality of the finished product, which includes equipment qualification.
- §211.110: Outlines the need for validated methods to ensure batch uniformity and integrity of drug products.
- FDA Guidance Documents: The FDA provides several guidance documents that elaborate on the expectations for equipment and process validation, including the General Principles of Software Validation and the Guidance for Industry on Process Validation: General Principles and Practices.
EMA (European Medicines Agency)
- EudraLex – Volume 4 – GMP Guidelines: The EU GMP guidelines provide detailed requirements for the qualification of equipment and validation of processes in Part 1 (Basic Requirements for Medicinal Products) and Annexes.
- Annex 15: Qualification and Validation:
- Installation Qualification (IQ): Should demonstrate that the equipment has been installed in accordance with the manufacturer’s specifications and requirements.
- Operational Qualification (OQ): Should demonstrate that the equipment operates as intended throughout all anticipated operating ranges.
- Performance Qualification (PQ): Should demonstrate that the equipment consistently performs according to the process requirements in actual production.
Key Sections in Annex 15:
- Section 3.2: States that a risk-based approach should be used to determine the extent of validation and qualification efforts.
- Section 4.1: Emphasizes the importance of documented evidence that facilities, systems, and equipment have been installed correctly (IQ), operate correctly (OQ), and perform effectively and reproducibly (PQ).
Key Regulatory Expectations
Some of the key regulatory expectations when auditing IQ, OQ, and PQ include:
1. Risk-Based Approach
Regulatory bodies emphasize using a risk-based approach to determine the scope and depth of IQ, OQ, and PQ activities. This involves identifying and mitigating risks that could affect product quality and patient safety.
READ MORE: Quality Risk Management in the Pharmaceutical Industry
2. Comprehensive Documentation
Detailed documentation is required at each stage of the qualification process. This includes protocols, test results, calibration records, deviation reports, and final summary reports.
3. Change Control
A formal change control process should be in place to manage any modifications to equipment, systems, or processes that may impact qualification status.
4. Periodic Review and Requalification
Periodic reviews and requalification should be conducted to ensure that equipment and systems remain qualified throughout their lifecycle.
5. Training
Personnel involved in qualification activities should be adequately trained and qualified to perform their duties.
FAQ
When Should IQ, OQ, and PQ Be Performed?
IQ: Conducted during the initial setup and installation of new equipment or systems, or after relocation or major maintenance.
OQ: Performed after successful completion of IQ and before the equipment or system is used for actual production. It can also be required after significant changes or upgrades.
PQ: Conducted after successful completion of both IQ and OQ, under actual production conditions. It can also be performed periodically as part of routine requalification.
How Is PQ Different From OQ?
While OQ focuses on verifying that the equipment or system operates correctly under controlled conditions, PQ ensures that it performs effectively in the actual production environment. PQ tests are conducted under real-world conditions, often with actual production materials and processes.
What Are the Acceptance Criteria for IQ, OQ, and PQ?
Acceptance criteria are predefined standards or specifications that test results must meet to be considered successful. These criteria should be clear, measurable, and based on regulatory requirements, manufacturer’s specifications, and internal quality standards.
How Often Should Requalification (Revalidation) Be Performed?
Requalification should be performed periodically based on a risk assessment, regulatory requirements, and internal policies. It is typically required after significant changes to equipment or processes, major maintenance, or deviations indicate a potential impact on product quality.
Can IQ, OQ, and PQ Be Combined Into a Single Document?
In some cases, especially for less complex equipment or systems, IQ, OQ, and PQ activities can be combined into a single document known as an integrated qualification protocol. However, for more complex or critical systems, separate documents are recommended to ensure thoroughness and clarity.
Conclusion
The validation processes of IQ, OQ, and PQ in GMP environments are fundamental in pharmaceutical manufacturing. They ensure that equipment is installed accurately, operates within designated limits, and performs consistently under actual production conditions.
These qualifications are essential for meeting regulatory standards and ensuring the safety and efficacy of pharmaceutical products. By rigorously applying these protocols, manufacturers uphold quality and reliability, building trust among healthcare providers, regulators, and patients.















