Failing to properly manage Out-of-Trend (OOT) can lead to costly recalls, regulatory scrutiny, and compromise patient safety. OOT results act as early warnings of potential issues in manufacturing or product stability, making their timely detection and management crucial.
OOT results indicate deviations from historical data trends, even if they remain within specification limits, serving as early warnings of potential shifts in manufacturing or product stability.
This article will explore the causes, detection methods, and management of OOT results within the framework of Good Manufacturing Practice (GMP). It highlights the importance of using statistical tools for trend analysis, distinguishing OOT from Out-of-Specification (OOS) and Out-of-Expectation (OOE) results, and meeting regulatory expectations.
What Are OOT Results?
OOT results refer to analytical data that, while still within the specified acceptance criteria, deviate from the expected or historical trend over time. These results can signal potential issues with the manufacturing process, equipment, or even the analytical method itself.
OOT results differ from Out-of-Specification results, as they do not exceed set limits, but their deviation from the normal pattern may indicate underlying concerns that could lead to future non-conformities. Proper investigation and corrective actions are essential to identify the root cause and ensure ongoing process control and product quality.
Common Causes of OOT Results
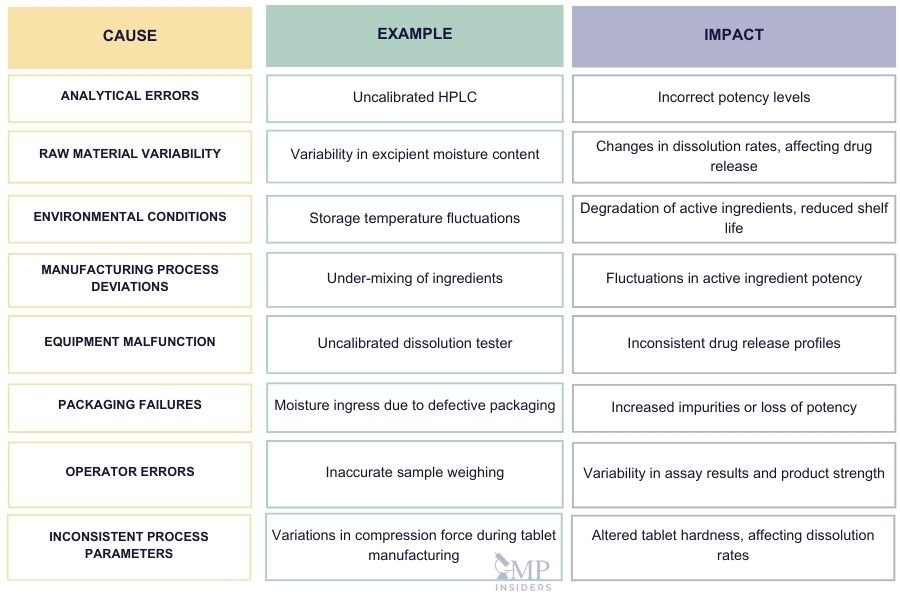
OOT results can arise from various factors throughout the manufacturing, testing, and storage of pharmaceutical products. These deviations, though still within specification limits, signal potential inconsistencies that may affect product quality over time. Understanding the common causes of OOT results is essential for timely intervention and maintaining control over the manufacturing process. Below are some of the primary causes of OOT results, along with examples.
- Analytical Errors: Miscalibrated instruments, errors in sample handling, or deviations from standard testing methods can produce inaccurate results, leading to OOT.
- Example: An improperly calibrated HPLC might generate inconsistent results over time, showing false trends in product performance.
- Raw Material Variability: Even when materials meet specification limits, minor variations between suppliers or batches can affect the final product’s quality.
- Example: Slight differences in the moisture content of an excipient can cause significant changes in tablet dissolution rates, impacting drug efficacy.
- Environmental Conditions: Factors such as temperature, humidity, and light exposure during manufacturing, testing, or storage can affect product stability, causing OOT trends.
- Example: Products stored at temperatures higher than recommended may degrade faster, leading to increased impurity levels during stability testing.
- Manufacturing Process Deviations: Inconsistent process parameters like mixing times, compression forces, or drying conditions can introduce variability in the final product, leading to OOT results.
- Example: Slight under-mixing of ingredients may result in uneven distribution of active ingredients, causing potency levels to fluctuate over time.
- Equipment Malfunction: Equipment that is malfunctioning or not properly maintained can affect testing accuracy or production consistency, leading to OOT results.
- Example: A dissolution tester that is not properly calibrated might produce varying drug release profiles, resulting in unreliable data trends.
- Packaging Failures: Defective packaging can expose the product to environmental elements, compromising its stability and leading to OOT results over time.
- Example: Packaging that fails to protect against moisture ingress can lead to product degradation, causing impurities or loss of potency during stability studies.
What Is the Difference Between OOS, OOE, and OOT Results?
Good Manufacturing Practice (GMP) guidelines emphasize the need to fully understand processes related to OOS, OOE, and OOT results. It’s no longer enough to be within specification limits while being out of statistical control, as this increases the risk of falling out of specification at some point.
While OOS signifies a result clearly outside acceptable limits, OOT and OOE are focused on detecting and predicting early trends that may lead to OOS situations. This ensures proactive quality control and product consistency.
- Out-of-Specification (OOS): Results that exceed predefined specification limits for critical quality attributes, indicating a failure.
- Out-of-Expectation (OOE): Results that do not exceed specification limits but are inconsistent with previous data or expected trends. OOE often points to variations in process or equipment performance. While not critical, addressing it early helps avoid OOS or OOT issues later on.
- Out of Trend (OOT): Results that deviate from historical trends but remain within specification limits. OOT is often detected during stability testing and may indicate early signs of quality issues.
Key Differences
- OOT helps catch issues before they become OOS.
- OOS directly affects product compliance and usually leads to batch rejection.
- OOE indicates unusual and unexpected results that may require investigation.
SEE ALSO: OOS vs OOT vs OOE Results in GMP
OOT Investigation Phases
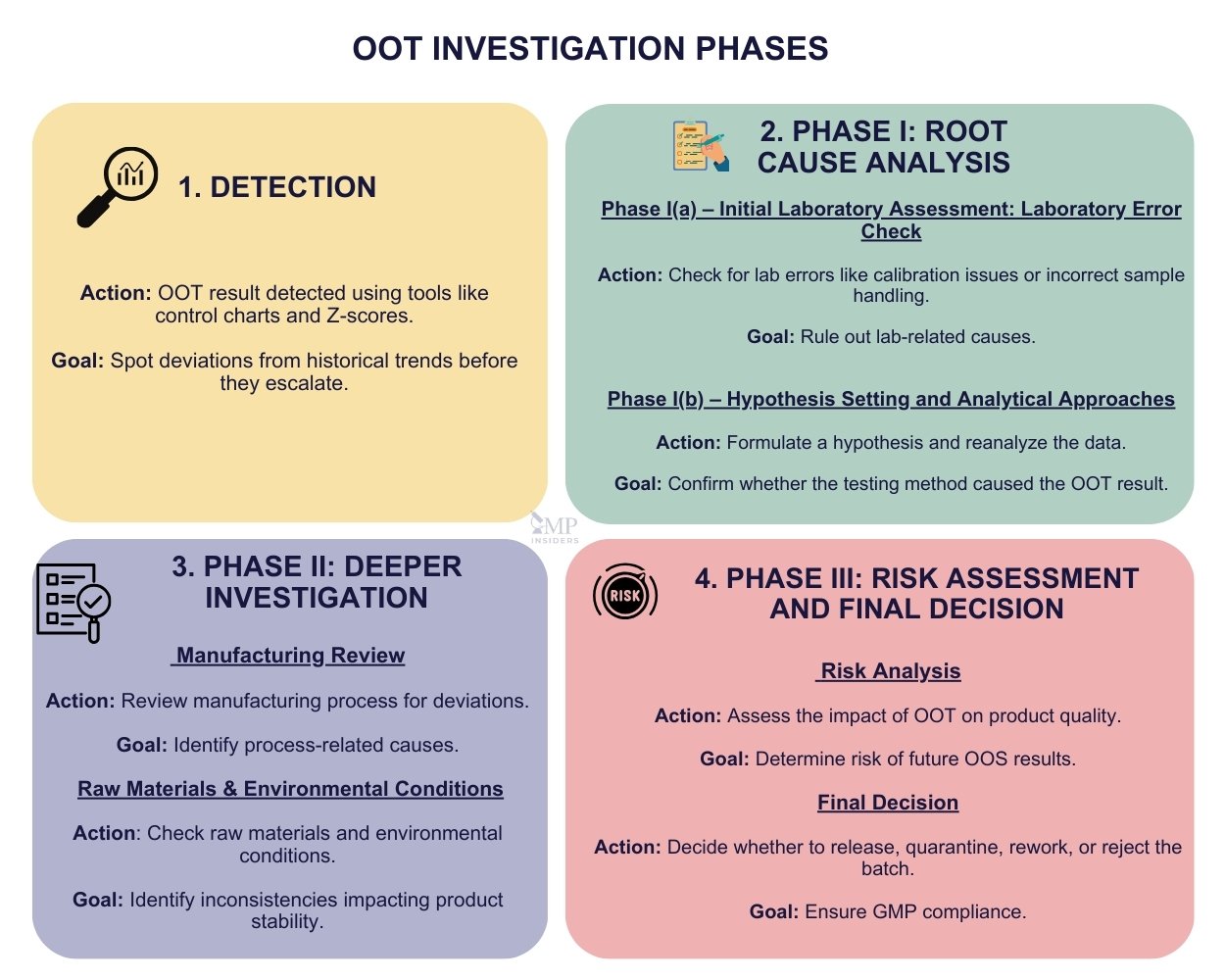
When an Out-of-Trend result is detected, it initiates a structured investigation to identify the root cause and implement corrective actions to maintain product quality and prevent future deviations. In general, OOT investigations start similarly to Out-of-Specification investigations, focusing on identifying any immediate errors or anomalies before moving to deeper root cause analysis. Below, the phases of OOT investigation are outlined, highlighting the systematic approach to detecting and addressing these trends.
Detection
The detection of an Out-of-Trend result triggers a structured investigation process aimed at identifying the root cause and implementing corrective actions. This ensures that product quality is maintained and future deviations are prevented. OOT detection typically involves:
- Control Charts: These charts monitor the behavior of key quality attributes over time. OOT results are flagged when data points deviate consistently from established historical trends, indicating potential emerging issues.
- Z-Score Analysis: This statistical method identifies subtle deviations from the expected norm. Z-scores flag unusual data points that are within specification limits but deviate significantly from the average, signaling a potential shift in process or product performance.
By detecting OOT results early, manufacturers can initiate investigations and corrective actions before product quality is compromised, allowing for a more proactive approach to quality control. This phase is critical because it enables real-time monitoring of product performance over time, ensuring that trends are identified before they escalate into more serious issues, like Out-of-Specification results.
Phase I: Root Cause Analysis
Root Cause Analysis (RCA) is a crucial part of Phase I in an OOT investigation and aims to identify the underlying cause of the observed deviation. The process ensures that corrective measures can be applied to the root cause and prevent recurrence.
Phase I(a) – Initial Laboratory Assessment: Laboratory Error Check
This phase starts with a predefined questionnaire covering the most common and probable laboratory mistakes that could arise during analysis. Every laboratory should create its own questionnaire based on the lab’s specific activities and tests. Key areas include checking for issues with instrument calibration, system suitability tests (SSTs), analyst performance, sample preparation, and adherence to the validated analytical method.
If any errors are found during this step, the necessary corrections are made, and the sample is reanalyzed. The purpose of this phase is to rule out any errors that may have occurred due to human or equipment malfunction before moving to a more in-depth analysis.
Phase I(b) – Hypothesis Setting and Analytical Approaches
If no lab errors are identified, the investigation delves further into every aspect of the analytical process to uncover any potential root cause that may be less apparent. The goal is to confirm whether the analytical method was applied correctly and remains suitable for the analysis. A hypothesis needs to be set, and all future analyses should be conducted according to the hypothesis plan.
This involves re-analyzing the sample using the same or a different validated method to verify consistency.
Phase II: Deeper Investigation
Phase II is a deeper investigation into potential non-laboratory causes of the OOT result. If the initial laboratory checks from Phase I don’t identify the issue, Phase II focuses on examining manufacturing processes, raw materials, environmental factors, and overall product or batch quality.
An OOT investigation requires collaboration across multiple departments, including Quality Control (QC), Quality Assurance (QA), Manufacturing, Engineering, and R&D. Each team plays a critical role in understanding different aspects of the issue and providing insights on how to resolve it.
Roles:
- QC: Primarily responsible for detecting the OOT and performing necessary testing.
- QA: Oversees the investigation, ensuring adherence to GMP requirements and that CAPA is properly implemented.
- Manufacturing/Engineering: Provides insights on equipment performance, raw materials, and process operations.
- R&D: Evaluates the formulation design, material specifications, process development, and analytical method development that may have influenced the OOT result.
Cross-functional collaboration improves the speed and effectiveness of OOT investigations, helping to pinpoint issues that might not be apparent from a single department’s perspective.
- Raw Material Variability: Investigate whether any inconsistencies in raw materials, even if within specification, may have contributed to the OOT result. Variability in supplier quality can have subtle but significant effects on product stability.
- Environmental Conditions: Review environmental factors like temperature and humidity during manufacturing, storage, or testing to identify any deviations that might explain the OOT result.
- Process Deviations: Examine production records for any deviations in key process parameters (e.g., mixing times, temperatures, pressures). Even minor adjustments in these parameters can cause gradual changes in product characteristics, which might appear as OOT trends.
- Trend Analysis: Compare the OOT result against historical data to determine whether it represents a long-term trend or a one-off anomaly.
- Cross-Batch Comparison: Review data from other batches to assess whether the OOT trend is isolated to one batch or if it indicates a broader issue affecting multiple lots.
Phase III: Risk Assessment and Final Decision
In Phase III, a risk-based approach is applied to assess the impact of the OOT result on the product’s quality, safety, and efficacy. This phase involves:
- Risk Prediction: Predicting the likelihood of obtaining an Out-of-Specification result by the product’s expiry date.
- Final Review and Decision: A thorough evaluation is made by the team, and decisions are taken regarding the batch’s release, possible rejections, or any necessary changes to the manufacturing or testing processes. This phase ensures that the final product complies with GMP and regulatory standards.
Key Outcomes
The investigation process leads to several important objectives:
- Reduced Risk: Prevents the likelihood of future deviations and ensures compliance with GMP standards.
- Improved Understanding: Offers deeper insight into process performance, allowing for continual improvement.
- Ensured Product Quality: Guarantees that the final product meets the quality specifications required for safe and effective use.
This phased approach ensures that each OOT result is properly investigated, and any root causes are addressed before the product is released.
CAPA in OOT Investigations
CAPA (Corrective Actions and Preventive Actions) ensures that the root causes of OOT results are addressed effectively and long-term measures are implemented to prevent recurrence. This process is essential for maintaining consistent product quality and compliance with regulatory standards.
Corrective Actions
These are immediate steps taken to resolve the identified issue that caused the OOT result. Examples include:
- Process Adjustments: If the OOT result was linked to a deviation in manufacturing processes (e.g., mixing time, compression force), immediate recalibration or modification of process parameters is required to restore consistency.
- Revised Standard Operating Procedures (SOPs): If the OOT investigation reveals procedural gaps, SOPs are updated to address the specific issue. This helps standardize procedures and reduce the likelihood of future deviations.
- Raw Material Handling: If raw material variability is identified as the cause, stricter controls are introduced to ensure uniform quality. This can involve more rigorous supplier audits, tighter material specifications, or more frequent testing of incoming materials.
Preventive Actions
Preventive actions aim to systematically improve processes and avoid similar OOT results in the future:
- Enhanced Monitoring: Increasing the frequency of monitoring critical process parameters (e.g., temperature, humidity, material quality) ensures that any deviation is identified early. This proactive approach helps detect trends before they become significant issues.
- Staff Training: Human error is a common contributor to OOT results. By conducting focused training, employees gain a clearer understanding of processes and SOPs, reducing errors and ensuring consistency in quality control.
- Trend Analysis Automation: Implementing or upgrading data tracking systems like Laboratory Information Management Systems (LIMS) automates trend analysis and minimizes human error. Automated systems can detect early deviations, helping to prevent OOT results from escalating into more severe issues.
The Role of OOT Monitoring in Product Stability
Stability studies evaluate how factors like temperature and humidity affect key stability indicating parameters such as potency, dissolution, and impurity levels. OOT monitoring is critical for assessing the long-term stability of pharmaceutical products.
By analyzing trends in stability data, manufacturers can detect subtle changes before they escalate into significant quality failures. OOT analysis compares current data against historical trends to ensure the product behaves as expected. Deviations may signal potential degradation or other quality concerns.
For example, identifying an OOT result related to potency loss at an early stage allows manufacturers to implement corrective actions, preventing the product from reaching an Out-of-Specification condition. This proactive approach ensures the product’s quality, safety, and efficacy are preserved throughout its shelf life. Without OOT monitoring, these early warning signals could be missed, leading to more severe quality issues down the line.
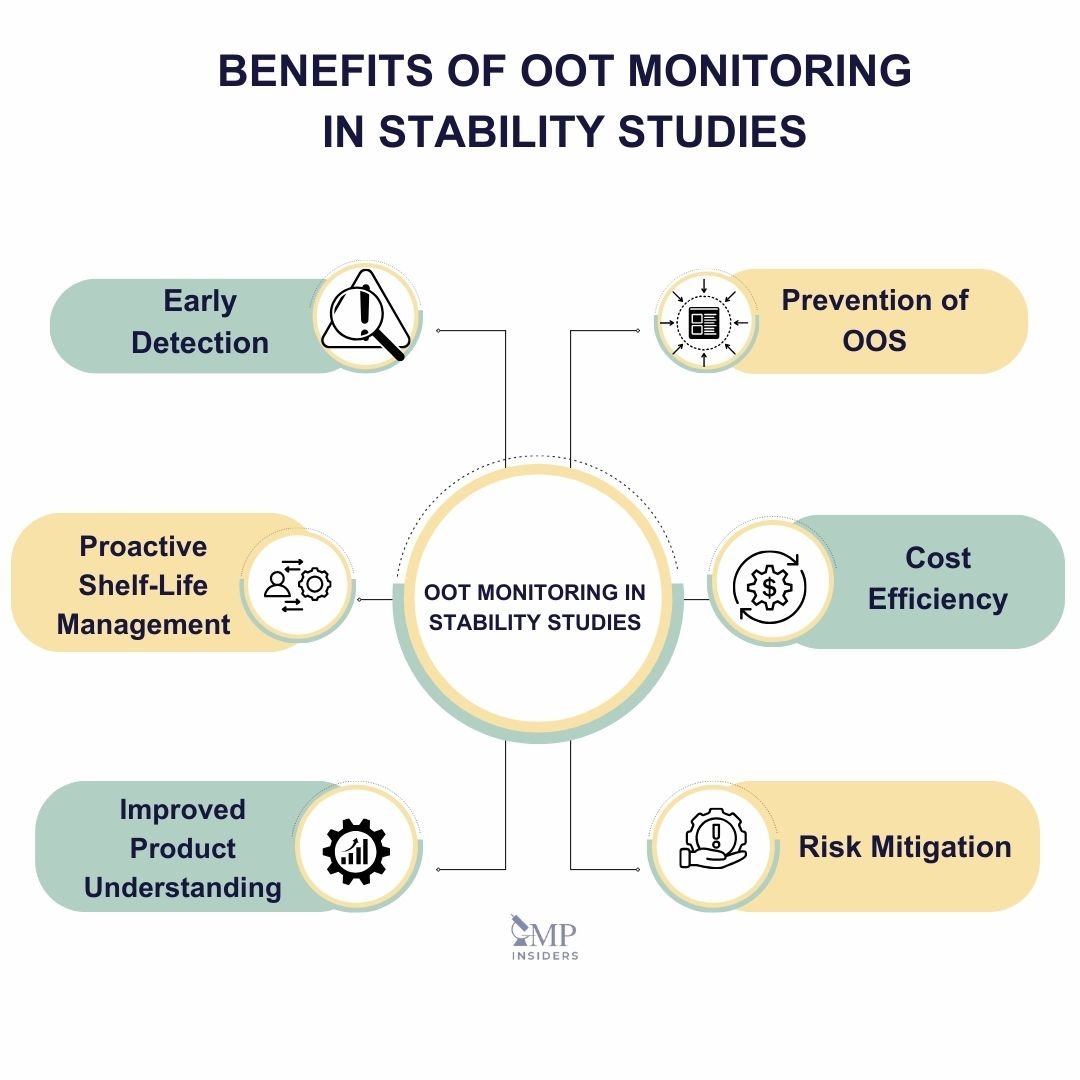
Key Benefits of OOT Monitoring in Stability Studies
- Early Detection of Potential Issues: Detects small deviations that could indicate underlying stability problems before they become critical.
- Prevention of OOS: Identifies trends that may lead to Out-of-Specification results, allowing for corrective actions before compliance issues arise.
- Proactive Shelf-Life Management: Facilitates adjustments in formulation, packaging, or storage conditions to maintain product quality over its intended lifespan.
- Cost Efficiency: Prevents larger failures by addressing issues at the trend stage, reducing the financial burden of rework or recalls.
- Improved Product Understanding: Continuous monitoring helps manufacturers better understand how external factors influence product stability, leading to enhanced future formulations and processes.
- Risk Mitigation: Minimizes the risks associated with product instability, protecting brand reputation and ensuring patient safety.
SPC Tools and Methods for Detecting OOT Results
Statistical Process Control (SPC) plays a crucial role in ensuring pharmaceutical processes remain within control, helping to identify OOT results early on. By using SPC tools such as control charts and process capability studies, manufacturers can detect deviations from expected trends before they escalate into OOS results. This proactive approach supports continuous process monitoring and improvement, reducing variability and ensuring product consistency.
SPC focuses on understanding process variability, which can be due to either common or special causes. By distinguishing between these causes and controlling special cause variability, manufacturers can maintain stable processes. The tools and methods involved, including variable and attribute data control charts, help identify patterns or shifts in the data that signal potential risks to product quality.
In addition, process capability indices (Cp, Cpk, Pp, Ppk) provide insights into how well a process can stay within specification limits. By measuring both the spread and centering of the process, these indices highlight areas needing improvement to avoid OOT results. The widely used 3-sigma approach, with its control limits set at three standard deviations from the process mean, is another key method in SPC. It enables early detection of process instability, preventing quality issues from arising.
Regulatory Guidelines for OOT
The Good Manufacturing Practices (GMP) guidelines outline strict monitoring requirements for product quality throughout a product’s lifecycle. The EU GMP Volume 4 Part I specifically addresses trend evaluation in Product Quality Review (PQR) to highlight trends and identify opportunities for process improvement.
SEE ALSO: Product Quality Review (PQR) in GMP
Similarly, Chapter 6 emphasizes that data from batch release testing (e.g., assay, dissolution, impurities) and environmental controls should be recorded to enable trend evaluation. Any Out-of-Trend result must be promptly addressed and investigated, and significant negative trends from stability testing must be reported to the appropriate regulatory authorities. Stability data trending is viewed as a proactive approach to detect changes over time and predict future trends in product quality.
Regulatory inspections have highlighted several deficiencies related to trend analysis, especially in terms of in-process control (IPC) data that are not consistently monitored for trends, or trend monitoring being limited to graphical data presentation without proper statistical analysis. In some cases, significant negative trends during stability studies were not reported to the authorities, only focusing on Out-of-Specification results, which have been flagged as a major compliance issue.
This demonstrates that regulators expect effective use of statistical tools for trend analysis to proactively manage product quality, with an emphasis on comprehensive trending of both OOT and OOS results, along with the necessary follow-up actions when trends indicate a risk to product stability or compliance.
FAQ
What Should Be Done if an OOT Result Is Confirmed as a Laboratory Error?
If an OOT result is confirmed as a laboratory error (e.g., equipment malfunction or operator error), the sample should be re-tested. The new result will replace the original, invalidated data in the records, as the lab error has been verified. Additionally, the erroneous data must be fully documented as part of the investigation to ensure transparency and compliance with GMP standards.
How Does OOT Detection Impact Stability Protocols?
OOT detection might require changes to stability protocols, such as increased frequency of testing, tighter control of environmental conditions, or adjustments to sampling strategies to ensure long-term product stability.
Is OOT Monitoring Necessary for Every Batch?
OOT monitoring is particularly critical for batches undergoing stability testing, but ongoing production batches should also be periodically reviewed to ensure consistent quality and identify deviations from historical data trends. Every OOT result should be carefully evaluated against Product Quality Reviews (PQRs), where established trends are defined, to determine whether the result signals a potential issue requiring further investigation or corrective action.
Conclusion
Managing Out-of-Trend results is a critical aspect of pharmaceutical quality control, serving as an early warning system that helps manufacturers address potential quality issues before they escalate into more serious problems, such as OOS results.
Through proactive detection and investigation of OOT results, companies can maintain product quality, safety, efficacy, and shelf life, ensuring compliance with current Good Manufacturing Practices (cGMP) and avoiding costly recalls or regulatory scrutiny.
Effective OOT investigations require a cross-functional approach, drawing on the expertise of Quality Control, Quality Assurance, R&D, Manufacturing, and Engineering teams. The use of statistical tools for trend analysis, along with a thorough understanding of OOT, OOS, and OOE, helps to build a more robust and resilient quality management system.















