Cleanrooms are used in pharmaceutical manufacturing to provide controlled environmental conditions that prevent contamination from affecting product quality. They are designed and qualified to maintain defined limits for airborne particulate and microbiological contamination through appropriate filtration, airflow design, pressure differentials, and controlled environmental parameters.
Ongoing control is demonstrated through monitoring, trending, maintenance, and documented responses to adverse results, ensuring the area remains suitable for the intended operations across its lifecycle.
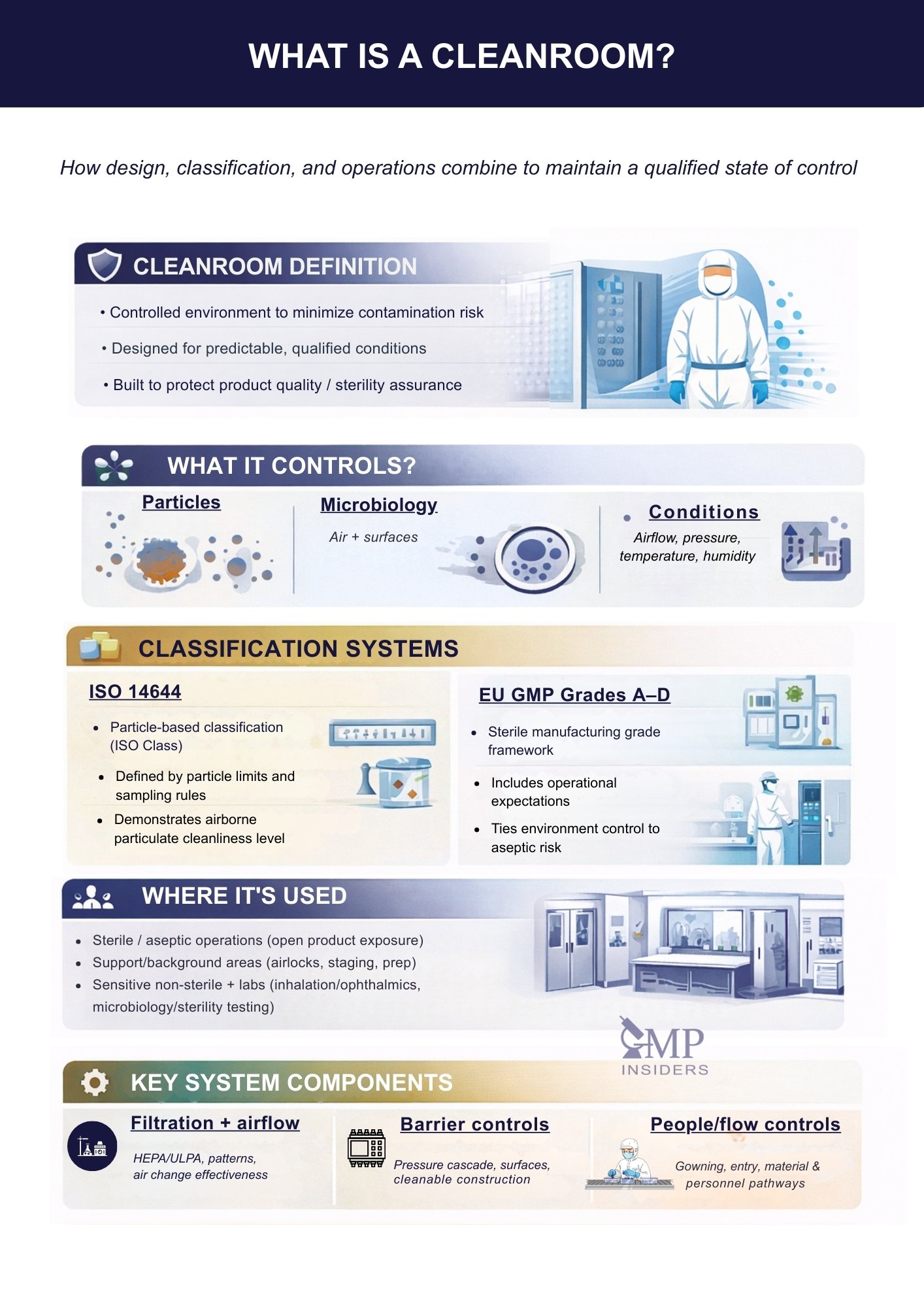
This article explains what a pharmaceutical cleanroom is, what environmental parameters it controls, how ISO 14644 classes and EU GMP Grades relate, where cleanrooms are used in pharma operations, and which design and operational elements are typically expected to maintain the area in a qualified state of control.
What Is a Cleanroom?
A cleanroom is a controlled environment designed to minimize and manage contamination that could affect the safety, quality, or performance of pharmaceutical products. Unlike ordinary rooms, cleanrooms are engineered to control airborne particles, microbial contamination, and environmental parameters such as airflow, temperature, humidity, and pressure.
These conditions are defined, qualified, and continuously verified to remain within validated limits.
In practical terms, a cleanroom is a space in which the environment is predictable and scientifically controlled. Air is filtered through HEPA or ULPA systems; airflow patterns are designed to remove and dilute contaminants; surfaces are constructed from materials that do not shed particles; and the movement of people and materials is carefully controlled.
Every design and operational element exists for one reason: to reduce the risk of contamination to an acceptable, scientifically justified level.
Regulatory frameworks such as EU GMP, FDA cGMP, and ISO 14644 define how cleanrooms must be designed, classified, operated, and monitored.
Cleanroom performance must be demonstrated under defined conditions. For this reason, qualification and monitoring activities distinguish between “at rest” and “in operation” states.
At rest means the installation is complete, the equipment is installed and operating, and no personnel are present. In operation refers to the condition in which the area is functioning as intended, including the presence of personnel and routine activities.
The objective is to demonstrate that the cleanroom remains under control under process-relevant conditions. Classification and qualification results are therefore interpreted together with operational controls, including personnel practices, material flows, interventions, cleaning and disinfection effectiveness, and environmental monitoring performance.
Where a process involves open product exposure or critical aseptic manipulations, evidence of control in operation is particularly important, as personnel and activities are the dominant sources of contamination.
| Cleanroom feature | What it controls | How control is achieved | How it’s demonstrated (typical evidence) |
|---|---|---|---|
| HEPA/ULPA filtration | Airborne particulate load | Terminal filtration with defined supply/return design | HEPA/ULPA integrity test results, filter certification records |
| Airflow pattern | Contaminant removal and dilution at critical zones | Unidirectional or mixed airflow design; airflow balancing | Airflow visualization (smoke study), airflow velocity and uniformity test results |
| Air change rate / ventilation effectiveness | Build-up of particles during operation | Defined air volume, mixing, and dilution capacity | HVAC qualification data, recovery test results (where applied), performance trending |
| Pressure differentials | Ingress of less clean air | Pressure cascade between zones; door discipline and interlocks (where used) | Continuous DP monitoring trends, alarm logs, deviation records for excursions |
| Temperature & humidity control | Process stability, operator comfort, equipment performance | HVAC control loops and defined setpoints | Continuous monitoring records, calibration status of sensors, excursion investigations |
| Surfaces & construction finishes | Particle shedding, cleanability, microbial harbourage | Smooth, non-porous materials; sealed penetrations; minimized ledges and joints | Facility inspection findings, cleaning verification records, EM trend correlation |
| Cleaning & disinfection program | Microbiological contamination | Defined agents, frequencies, rotation and sporicidal use (as justified) | Approved SOPs, cleaning logs, disinfectant effectiveness or qualification data, EM trends |
| People controls (gowning & behavior) | Major source of particles and microbes | Controlled entry, gowning procedures, aseptic behaviors, training and qualification | Training and qualification records, gowning audits or observations, EM events linked to activities |
| Material & personnel flow | Introduction and spread of contamination | Airlocks, pass-throughs, segregated pathways, transfer disinfection | Flow maps and SOPs, access controls, transfer logs, deviations for flow breaches |
| Monitoring & response system | Ongoing “state of control” | Defined EM strategy, limits, review, investigation, and CAPA | Particle and viable monitoring results, trend reports, investigation records, CAPA effectiveness checks |
| Maintenance & periodic verification | Drift of critical systems over the lifecycle | Preventive maintenance, calibration, requalification triggers via change control | PM schedules, calibration certificates, requalification reports, change-control impact assessments |
What Does a Cleanroom Control?
A pharmaceutical cleanroom is designed to control specific environmental factors that can influence product quality, sterility assurance, and regulatory acceptability. Its purpose is not only to make the area visibly clean, but to maintain a scientifically defined level of environmental control that can be consistently demonstrated.
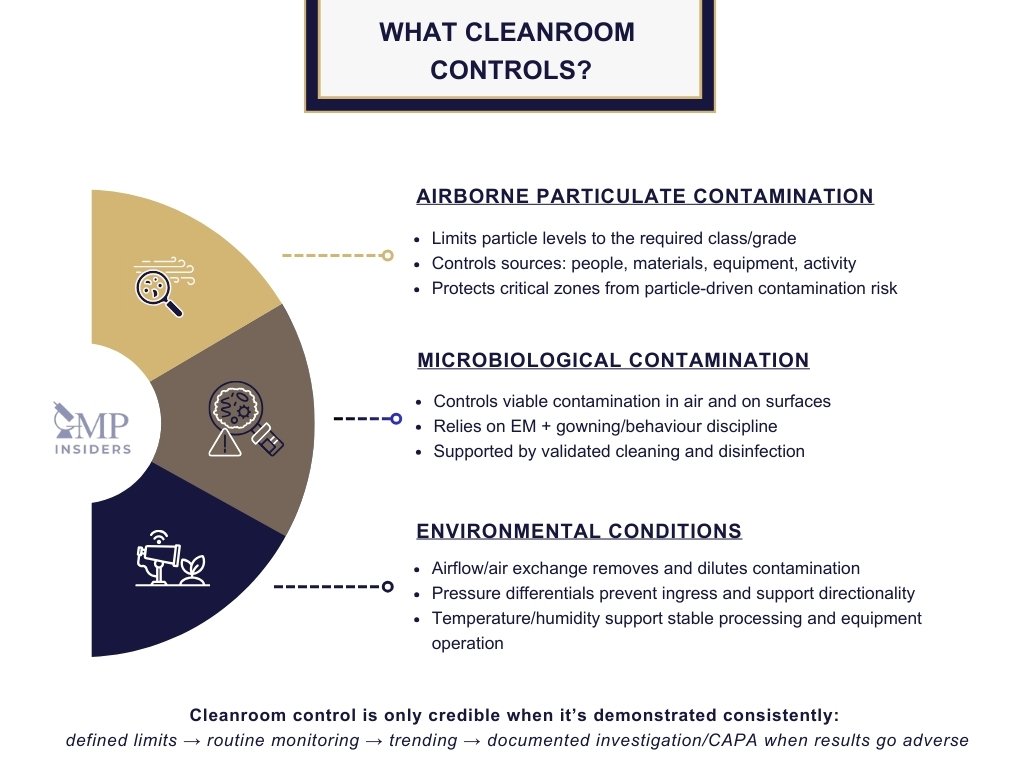
Airborne Particulate Contamination
Cleanrooms are primarily intended to limit the concentration of airborne particles originating from personnel, equipment, materials, and manufacturing activities. These particles can:
- Carry microorganisms into critical process areas
- Interfere with sensitive manufacturing operations
- Compromise product quality and sterility assurance
For this reason, cleanrooms operate under defined particle concentration limits that correspond to their classification and intended use.
Microbiological Contamination
Microorganisms represent a serious risk in pharmaceutical manufacturing, particularly in sterile product environments. Cleanrooms help control microbial presence through:
- Qualified environmental monitoring programs
- Strict personnel hygiene and behavior controls
- Validated cleaning and disinfection practices
This structured approach reduces the risk of bioburden and supports patient safety.
Environmental Conditions
Cleanrooms also control key physical environmental parameters that support both manufacturing performance and regulatory compliance, including:
- Airflow characteristics and air exchange rates: to dilute, remove, and continuously refresh air, reducing contamination buildup.
- Pressure differentials: to prevent contaminated air from entering cleaner zones and to maintain directional airflow between classified areas.
- Temperature and humidity: to support product stability, process consistency, operator comfort, and reliable equipment operation.
These parameters are defined during qualification, scientifically justified, and maintained through validated HVAC and control systems to ensure sustained environmental control.
Cleanroom Classes in Pharmaceutical Industry
Not all cleanrooms operate at the same level of control. In the pharmaceutical industry, environments are classified according to the degree to which particulate and microbiological contamination must be controlled, based on the risk and criticality of the process being performed.
ISO 14644 Cleanroom Classes
ISO 14644-1 provides an international classification system for airborne particles based on particle concentration. Cleanrooms are classified (commonly ISO 5 to ISO 8 in the pharmaceutical industry) according to the maximum allowable number of particles of defined sizes per cubic meter of air.
In practice, this classification enables manufacturers to define, measure, and demonstrate the cleanliness of their environment in a standardized, globally recognized manner.
SEE ALSO: Cleanroom Classification According to ISO 14644-1
EU GMP Grades A, B, C, and D
In addition to ISO classification, EU GMP Annex 1 cleanroom grades govern pharmaceutical sterile manufacturing:
- Grade A: Highest control level, typically for critical aseptic operations such as filling and open product exposure
- Grade B: Background environment for Grade A activities
- Grades C and D: Support areas used for preparation, less critical processing, or background environments where lower particle levels are acceptable
These grades are linked not only to particulate limits, but also to microbiological expectations and operational requirements.
SEE ALSO: GMP Cleanroom Classifications: Grade A, B, C, and D
How the Systems Relate
ISO classes provide a particle-based classification framework, whereas GMP Grades incorporate both particulate expectations and a broader sterility-assurance context within manufacturing. In pharmaceutical practice, they are often used together: ISO defines measurable particle control, while GMP Grades define how that control connects to process risk and regulatory expectations.
Where Are Cleanrooms Used in the Pharmaceutical Industry?
Cleanrooms are used wherever environmental control directly affects product quality, sterility assurance, or regulatory compliance. Their application is not limited to sterile manufacturing; they support a wide range of activities in which contamination must be minimized and controlled.
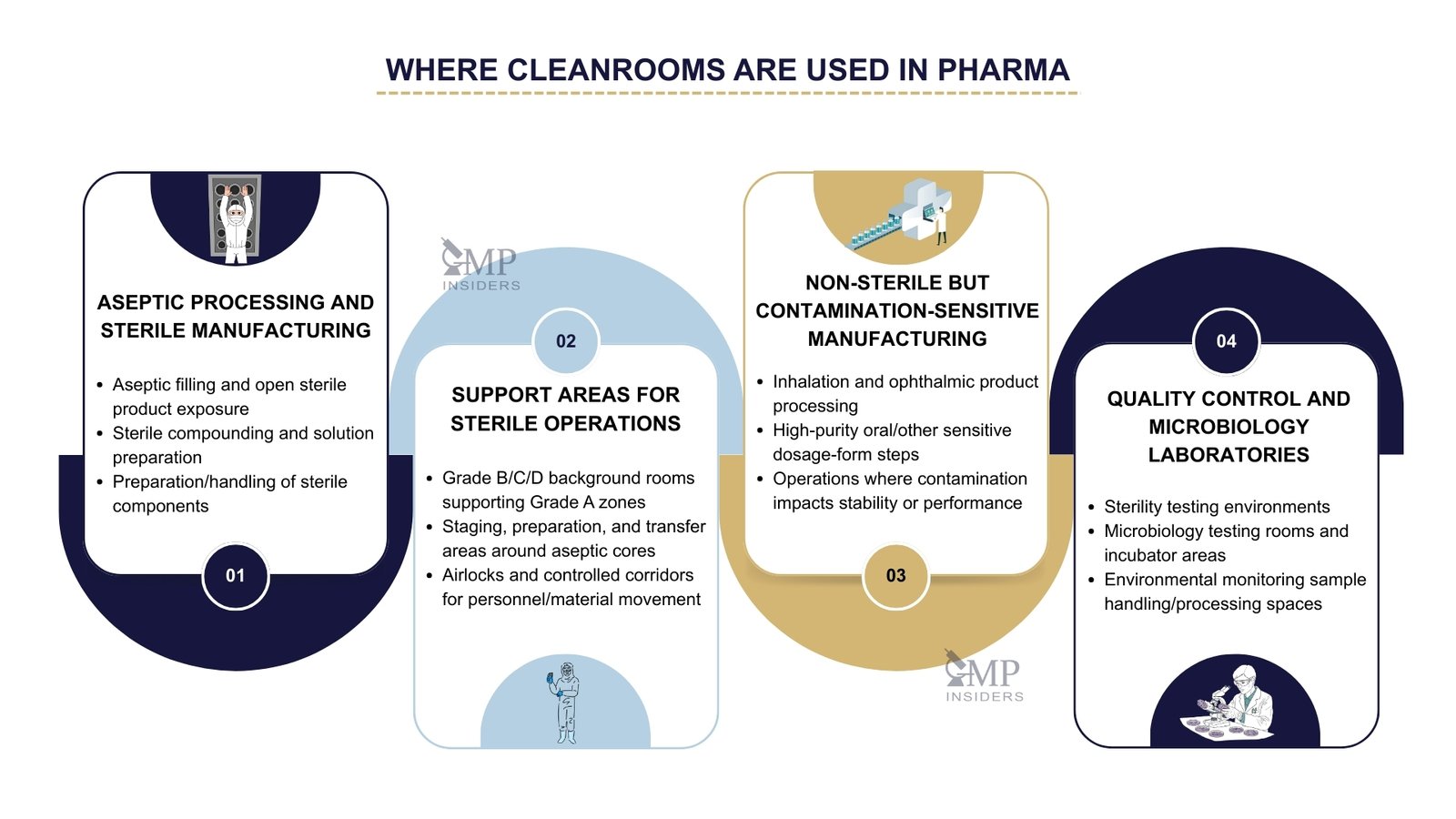
Aseptic Processing and Sterile Manufacturing
Cleanrooms play a central role in sterile product manufacturing, particularly in:
- Aseptic preparation and filling of injectable products
- Operations involving open product exposure
- Compounding of sterile formulations
- Preparation of sterile components and solutions
These environments require the highest level of environmental control because any contamination may directly reach the patient.
Support Areas for Sterile Operations
Surrounding rooms that support aseptic areas also operate as classified cleanrooms. These include preparation areas, staging zones, and background environments that provide controlled conditions for entry into critical processing spaces. Their role is to maintain a contamination barrier and to support the movement of products, components, and personnel under controlled conditions.
Non-Sterile but Contamination-Sensitive Manufacturing
Even where sterility is not required, controlled environments are often needed to:
- Protect products with microbial or particulate sensitivity
- Handle inhalation products, ophthalmics, or high-purity oral dosage forms
- Manage processes where contamination may affect product performance or stability
Here, cleanrooms help maintain consistency, reduce variability, and support GMP expectations.
Quality Control and Microbiology Laboratories
Many pharmaceutical laboratories also operate within controlled environments, particularly:
- Microbiological testing rooms
- Environmental monitoring laboratories
- Sterility testing areas
In these cases, the cleanroom supports the integrity and reliability of analytical results.
Key Components of a Cleanroom
A pharmaceutical cleanroom is not defined solely by its appearance or cleanliness; it is designed as a controlled system in which every component contributes to preventing contamination and ensuring regulatory compliance. Its effectiveness depends on how well its design elements, engineering controls, and operational practices work together.
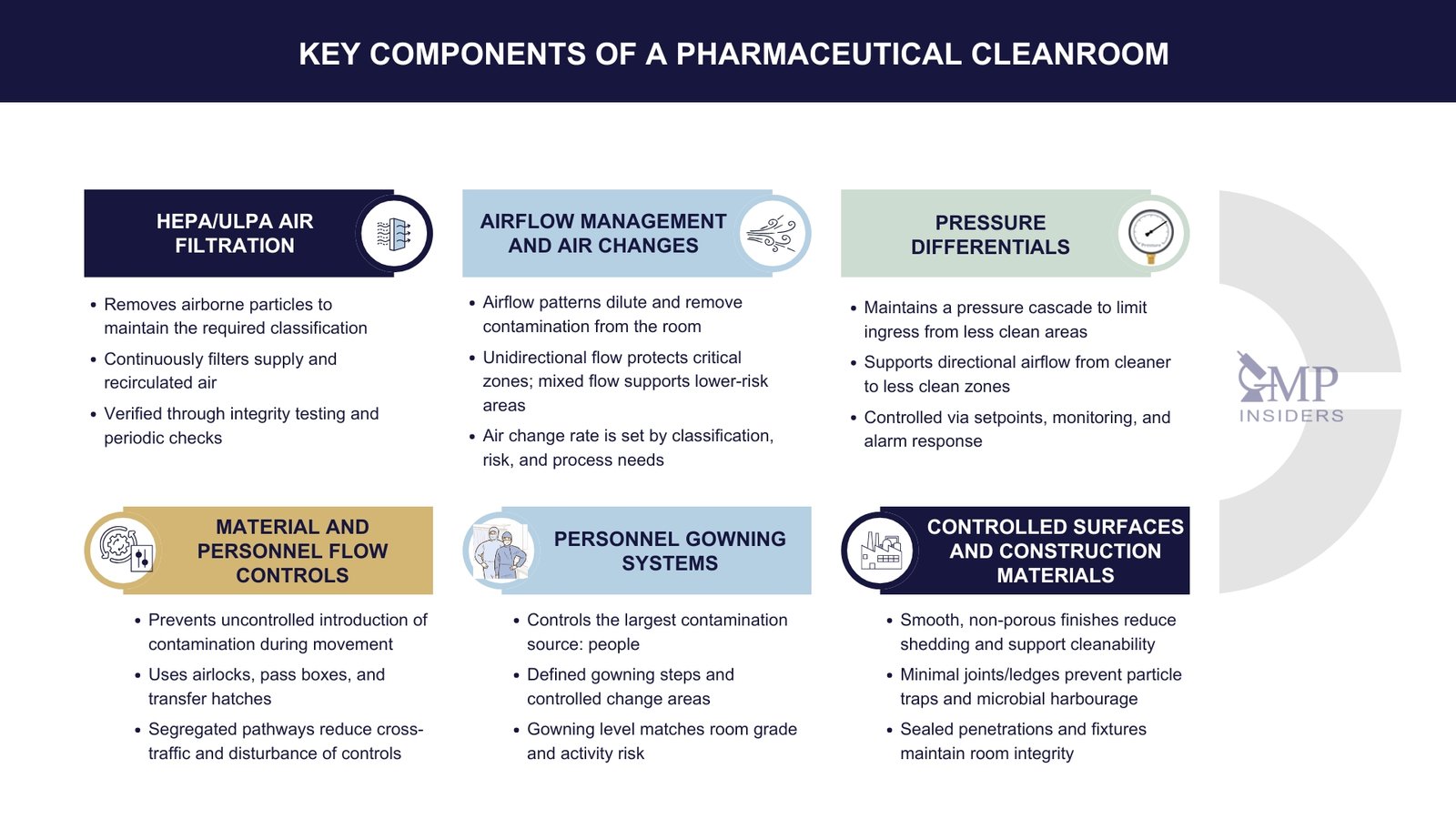
HEPA / ULPA Air Filtration
Cleanroom air is filtered through high-efficiency filtration systems:
- HEPA filters remove the majority of submicron airborne particles
- ULPA filters provide even higher efficiency where required
These filters continuously clean recirculated and supply air, helping maintain the room’s classification.
SEE ALSO: Type of HEPA Filters Used in the Pharmaceutical Industry
Airflow Management and Air Changes
Airflow patterns are engineered to remove and dilute contamination:
- Laminar (unidirectional) airflow is typically used in critical areas to sweep contamination away from product contact zones
- Turbulent or mixed airflow may be used in less critical spaces
The number of air changes per hour is determined by classification, risk, and process requirements.
Pressure Differentials
Positive pressure is maintained in most pharmaceutical cleanrooms to prevent contaminated air from entering controlled areas from surrounding spaces. Pressure gradients between zones of varying cleanliness help ensure that contamination flows outward to less-controlled areas rather than inward toward critical environments.
Controlled Surfaces and Construction Materials
Cleanrooms are constructed from materials designed to minimize shedding, prevent particle build-up, and withstand repeated cleaning and disinfection. This typically includes:
- Smooth, non-porous, easy-to-clean wall, floor, and ceiling finishes
- Minimal joints, ledges, and particle traps
- Sealed penetrations, doors, and fixtures
Personnel Gowning Systems
Since people are the largest contamination source, cleanrooms include structured gowning control:
- Defined gowning areas and procedures
- Use of gowns, masks, gloves, shoe covers, and head coverings
- Differentiated gowning requirements depending on room classification and activity
Material and Personnel Flow Controls
To reduce contamination risk, the movement of both people and materials is carefully managed through:
- Airlocks
- Pass boxes and transfer hatches
- Segregated flows and defined movement pathways
This prevents the uncontrolled introduction of contaminants into critical environments.
People, Procedures, and Behaviour in Cleanrooms
Technology and engineering controls define the structure of a cleanroom, but its performance in day-to-day pharmaceutical operations depends primarily on how personnel behave within it. Personnel are consistently identified as the primary source of contamination, which is why disciplined procedures, training, and controlled behavior are fundamental to cleanroom integrity.
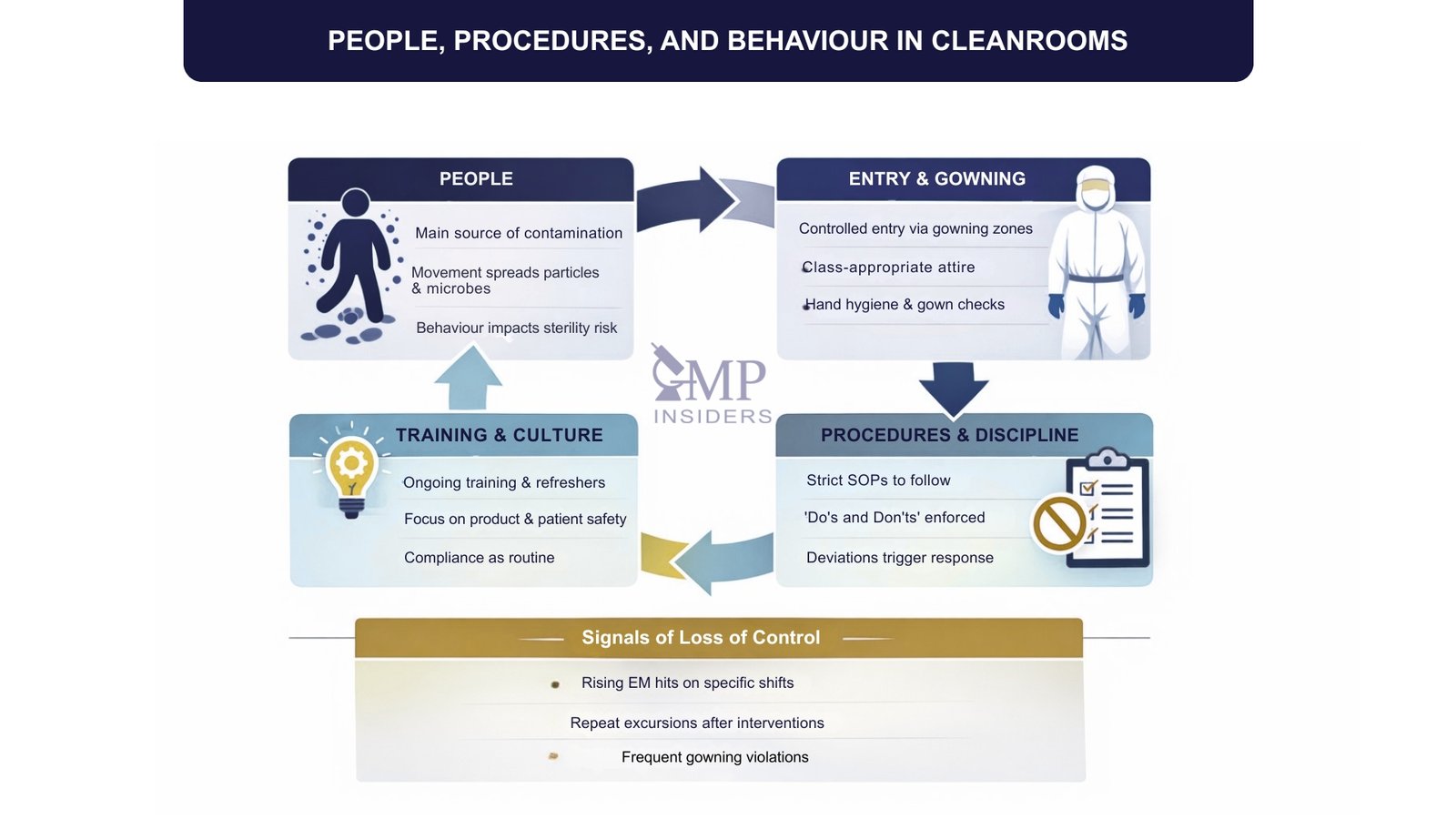
People as the Primary Contamination Source
Even when fully gowned, operators continuously shed particles and can introduce microorganisms through movement, poor hygiene, or incorrect practices. Simple actions such as rapid movement, unnecessary talking, improper glove use, or touching inappropriate surfaces can significantly increase the risk of contamination. Cleanroom operation, therefore, relies on trained personnel who understand not only what to do, but why it matters.
Gowning and Entry Procedures
Access to a cleanroom is controlled through structured entry systems and defined gowning procedures. This typically includes:
- Controlled change rooms and gowning zones
- Class-appropriate gowning (e.g., gloves, masks, coveralls, hoods, footwear)
- Strict rules for hand hygiene and gown integrity
- Verification that personnel are trained and authorized to enter
Gowning is part of the contamination control strategy and must be performed consistently and correctly.
SEE ALSO: Risk-Based Contamination Control Strategy (CCS)
Defined Procedures and Discipline
Activities performed inside cleanrooms are governed by approved SOPs that define:
- How operations are performed
- What personnel may and may not do
- How to handle materials, components, and equipment
- How to respond to deviations or contamination events
Adherence to procedures is critical because cleanroom performance must be predictable, repeatable, and defensible during inspection.
Training, Awareness, and Culture
Sustained cleanroom control requires more than initial instruction. Personnel must:
- Receive initial and periodic training
- Understand the impact of their actions on product and patient safety
- Maintain situational awareness and accountability
- Support a culture where compliance is normal behavior, not an obligation
In mature pharmaceutical environments, cleanroom discipline becomes part of organizational culture rather than a task enforced only by QA.
Cleanroom Operation and Maintenance
A cleanroom does not remain compliant simply because it has been well designed or initially qualified. Its performance depends on how it is operated, monitored, cleaned, and maintained throughout its lifecycle. In pharmaceutical manufacturing, cleanroom control must be continuously demonstrated rather than assumed.
Routine Environmental Monitoring
Environmental monitoring programs are established to verify that the cleanroom continues to operate within defined limits. These typically include:
- Airborne particle monitoring
- Viable airborne and surface microbiological monitoring
- Pressure differential checks
- Temperature and humidity monitoring
Monitoring is risk-based, aligned with classification and process criticality, and supported by defined alert and action limits. Results must be reviewed, trended, and acted upon where deviations occur.
Cleaning and Disinfection Practices
Cleanrooms require structured and validated cleaning and disinfection programs. These typically define:
- Cleaning frequencies for different classifications and areas
- Approved disinfectants, rotation strategies, and sporicidal use where necessary
- Methods and equipment to be used
- Documentation expectations and verification activities
The intent is not simply to clean, but to maintain a microbiologically controlled environment with scientifically justified practices.
Preventive Maintenance and Periodic Qualification
Mechanical systems such as HVAC and filtration are critical to cleanroom performance and must be maintained proactively. Activities include:
- Preventive maintenance schedules
- Periodic replacement or integrity testing of HEPA/ULPA filters
- Calibration of instruments and sensors
- Periodic requalification to verify continued compliance with classification requirements
These activities demonstrate that the environment continues to perform as validated initially.
| Area | What’s controlled | What you do routinely | Evidence you keep |
|---|---|---|---|
| Environmental monitoring | Particles, viable counts, DP, T/RH | Monitor to alert and action limits; trend and investigate adverse results | EM results, trend reports, deviations and CAPA |
| Cleaning & disinfection | Bioburden on surfaces and room | Grade-based frequencies; disinfectant rotation and sporicidal use (as justified) | Cleaning logs, disinfectant logs, verification records |
| Maintenance & requalification | HVAC, filters, and instruments performance | Preventive maintenance and calibration; HEPA integrity testing; periodic requalification | PM work orders, calibration certificates, integrity test results, requalification reports |
Regulatory Guidelines and Standards Relevant to Pharmaceutical Cleanrooms
Cleanroom requirements in pharmaceutical manufacturing are defined through GMP legislation and guidance, supported by harmonised standards that specify how classification, monitoring, qualification, and contamination control must be demonstrated.
EU GMP Framework
The foundational expectations start with EU GMP, which establishes the general requirements for premises, controls, documentation, and the pharmaceutical quality system.
EU GMP Part I / Part II
- Facilities and environmental conditions must be suitable for intended operations and prevent contamination/cross-contamination.
- Processes must be performed under controlled conditions with defined procedures and documented evidence.
- Deviations, changes, and maintenance impacts must be assessed, documented, and controlled within the PQS.
Link to guidance: EU GMP Part I / Part II
EU GMP Annex 1 (Sterile Medicinal Products)
- A documented Contamination Control Strategy (CCS) covering facility, HVAC, EM, cleaning/disinfection, personnel, and utilities.
- Defined cleanroom Grades (A/B/C/D) with expectations for airflow, monitoring, disinfection, gowning, interventions, and aseptic practices.
- Ongoing control demonstrated by environmental monitoring, trending, defined limits, and investigation/CAPA for adverse results.
Link to guidance: EU GMP Annex 1
EU GMP Annex 15 (Qualification and Validation)
- A lifecycle approach to qualification/validation with documented rationale, acceptance criteria, and traceability.
- Evidence that critical systems (e.g., HVAC) are qualified and remain fit for use, including periodic review/requalification triggers.
- Change control must include an impact assessment on the validated/qualified state.
Link to guidance: EU GMP Annex 15
ISO Cleanroom Classification and Monitoring Standards
While EU GMP defines regulatory expectations, ISO 14644 provides the international technical standard for classifying cleanrooms and verifying that airborne particulate control is achieved and maintained.
ISO 14644-1
- Classification based on airborne particle concentration at specified particle sizes.
- Defined sampling locations/volumes and acceptance criteria for stating an ISO Class.
Link to Guidance: ISO 14644-1
ISO 14644-2
- A plan for ongoing monitoring to provide evidence of continued compliance with the classification.
- Defined monitoring strategy, frequencies, and response when results indicate loss of control.
Link to Guidance: ISO 14644-2
ISO 14644-3
- Standardized test methods to verify cleanroom performance (e.g., airflow, recovery, leakage, etc., as applicable).
- Documented testing and criteria to support qualification and periodic verification.
Link to Guidance: ISO 14644-3
ISO 14644-4
- Requirements/guidance for cleanroom design and construction to achieve intended cleanliness and maintainability.
- Considerations for materials, finishes, access, layouts, and commissioning interfaces.
Link to Guidance: ISO 14644-4
ISO 14644-5
- Operational expectations: personnel practices, cleaning, gowning, material movement, and behaviors needed to maintain cleanliness.
Link to Guidance: ISO 14644-5
US Regulatory Expectations
For operations that supply or are inspected against US requirements, FDA cGMP provides the legal baseline for facility controls and contamination-prevention expectations.
21 CFR 210/211 (FDA cGMP)
- Facilities and controls must prevent contamination and mix-ups; procedures must be written and followed.
- Adequate environmental control and monitoring where needed for product quality.
- Robust investigation of failures/deviations and documented corrective actions.
Link to Guidance: 21 CFR 210/211
FDA Aseptic Processing Guidance
- Aseptic processing must be designed to minimize contamination risk through strict control of personnel and interventions.
- Expectations for environmental monitoring, airflow/HEPA integrity, disinfection, and media fill (APS) design/interpretation.
- Strong emphasis on demonstrating control in operation, not only “at rest.”
FAQ
Are Cleanrooms Needed in Every Pharmaceutical Manufacturing Facility?
No, not every pharmaceutical facility requires classified cleanrooms; however, any process in which environmental contamination can affect quality requires controlled conditions. The decision is based on risk, product type, route of administration, and manufacturing stage.
Oral solids may not require the same level of classification as sterile injectables, but still benefit from controlled environments. Cleanrooms are therefore applied proportionally rather than universally, in accordance with scientific principles and GMP expectations.
Can Cleanrooms Be Upgraded to a Higher Class Later?
Upgrading a cleanroom is possible but rarely straightforward. It may require redesigning HVAC capacity, modifying airflow patterns, introducing new filtration, and, at times, reconstructing walls, ceilings, and equipment layouts.
Operational procedures, gowning, and monitoring programs must also be elevated. Because of the complexity, most organizations design cleanrooms with lifecycle planning and future needs in mind.
Do Automated or Robotic Systems Reduce the Need for Strict Cleanroom Controls?
Automation reduces the risk of direct human contamination but does not eliminate the need for controlled environments. Equipment itself can generate particles, require maintenance, and introduce risks if not designed for hygienic use.
Controlled environments are still necessary to protect product exposure points and maintain sterility assurance. Automation complements contamination control but does not replace it.
What Happens if a Cleanroom Temporarily Loses Control (e.g., Pressure Failure)?
Loss of control requires immediate assessment based on risk, duration, and activities ongoing at the time. Operations may need to stop, impacted batches assessed, and recovery steps defined. Deviation investigation and CAPA typically follow to understand the root cause and prevent recurrence. Regulators expect evidence that companies handle such events systematically rather than reactively.
Do All Cleanrooms Require Unidirectional Airflow?
No, unidirectional (laminar) airflow is typically required only for the most critical operations in which direct product exposure occurs. Many pharmaceutical environments operate safely and in compliance using mixed- or turbulent-flow systems.
The airflow strategy must match risk, process sensitivity, and regulatory expectations. Over-engineering can be as problematic as under-controlling if not scientifically justified.
Can Cleanrooms Operate Continuously Without Shutdowns?
In theory, they can, but in practice, they require planned downtime for maintenance, deep cleaning, requalification, and system restoration. Continuous operation without structured control leads to a gradual decline in performance. Planned lifecycle management ensures stability and predictability. Regulators value demonstrated control over unrealistic “always running” environments.
Final Thoughts
Cleanrooms are more than controlled spaces or technical installations. They are integral to how pharmaceutical manufacturers demonstrate responsibility, scientific control, and regulatory maturity. Their role extends beyond particle counts and engineering performance; they support product quality, protect patients, and underpin trust in the medicines supplied to healthcare systems worldwide.
A compliant cleanroom is the result of many interconnected elements working together: thoughtful design, qualified systems, disciplined operation, trained personnel, and meaningful data review. When these are aligned, cleanrooms do not merely meet regulatory expectations; they actively strengthen the overall quality management system.
As technology advances and regulatory expectations continue to evolve, the industry is moving toward more innovative, more lifecycle-driven approaches to environmental control. However, the fundamental principle remains unchanged: a cleanroom must consistently provide a controlled, defensible environment that protects product quality and patient safety at every stage of manufacturing.















