Process validation is a critical aspect of Good Manufacturing Practices (GMP). It involves establishing scientific evidence that a manufacturing process consistently produces a product that meets predetermined quality requirements.
In this article, we will explore some key principles and guidelines covering process validation in GMP, its importance in manufacturing facilities, and ensuring safe and quality products, as well as the best practices to implement effective process validation strategies.
Understanding Process Validation
Process validation plays a crucial role in ensuring drug quality. It is based on the principle that quality cannot be assured solely through in-process and finished-product inspection or testing. Instead, quality should be built into the product, and the manufacturing process should be designed and controlled to consistently meet the desired quality attributes.
What is Process Validation?
“Process validation can be defined as documented evidence that the process, operated within established parameters, can perform effectively and reproducibly to produce a medicinal product meeting its predetermined specifications and quality attributes.”
The purpose of process validation is to ensure that the control strategy is sufficient for the process design and product quality. The validation process should include all strengths of the product as well as the production sites used for manufacturing the product.
One of the basic principles of quality assurance is that a medicine that is fit for its purpose should be manufactured. This includes taking into consideration the following:
- Quality, safety, and effectiveness are built into the product.
- Quality cannot be verified by in-process controls or finished product analysis.
- Each manufacturing process step is controlled to ensure that the finished product meets all defined quality attributes.
Why Do We Validate Our Processes?
Validation is essential for pharmaceutical manufacturing, but you are probably wondering why. What is the reason to validate the process if it can be controlled with in-process controls and analysis?
Some of the main thoughts and answers to this question include:
Key Principles of Process Validation
Process validation involves collecting and evaluating data from the process design stage through commercial production to ensure that the manufacturing process consistently produces a product meeting its predetermined specifications and quality attributes. Here are the key principles of process validation:
Process Understanding
Process understanding is the foundation of process validation. It involves acquiring knowledge about the critical process parameters, their impact on product quality, and the sources of process variability. Scientific and statistical methods, including risk assessment, experimental design, and process characterization, achieve this understanding.
Lifecycle Approach
Process validation follows a lifecycle approach, including three stages: process design, qualification, and continued process verification. The lifecycle approach ensures that process validation activities are integrated into the overall product lifecycle and are continuously monitored and improved.
Risk-Based Approach
A risk-based approach is crucial in process validation. It involves identifying and assessing potential risks associated with the manufacturing process and implementing appropriate controls to mitigate them. Risk assessment tools, such as Failure Mode and Effects Analysis (FMEA), are commonly used in this process.
Statistical Techniques
Statistical techniques are employed in process validation to analyze data and draw meaningful conclusions. These techniques help evaluate process performance, establish process capability, and detect process variability. Key statistical tools include:
- Histogram Distributions
- Statistical Process Control
- Process Capability
- Design of Experiments
- Measurement Systems Analysis
- Sampling Plans
Regulatory Requirements for GMP Process Validation
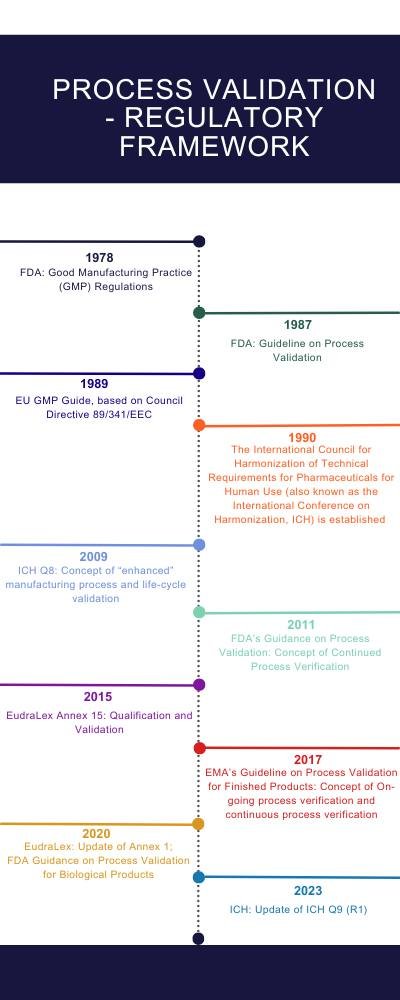
Regulatory authorities around the world have established guidelines to ensure that the manufacturing processes are well-controlled and capable of consistently producing quality products.
EMA
The European Medicines Agency (EMA) oversees the regulation of medicines within the European Union. EMA’s approach to process validation is detailed in its guidelines, with a significant document being EMA/CHMP/CVMP/QWP/70278/2012-Rev1, Corr.1, which outlines expectations for the validation of manufacturing processes. This guideline emphasizes a life-cycle approach, integrating quality by design (QbD), risk management, and continuous process verification to ensure processes are well-controlled and products meet quality standards.
Additionally, Annex 15 to the EU Guidelines for Good Manufacturing Practice (GMP) is critical for understanding specific requirements related to qualification and validation. Annex 15 provides detailed guidance on the principles of qualification and validation, essential for ensuring that manufacturing processes can consistently produce products that meet the intended specifications.
FDA
The U.S. Food and Drug Administration (FDA) provides comprehensive guidance on process validation for the pharmaceutical industry, detailed in the document titled “Guidance for Industry: Process Validation: General Principles and Practices.” This guidance outlines a three-stage process validation framework: Process Design, Process Qualification, and Continued Process Verification. The FDA emphasizes a science- and risk-based approach, encouraging manufacturers to use data from the entire product lifecycle to ensure and improve product quality.
ICH
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is pivotal in harmonizing regulatory expectations across regions, offering guidelines that span the entire pharmaceutical product lifecycle. The ICH guidelines, particularly Q8 (Pharmaceutical Development), Q9 (Quality Risk Management), and Q10 (Pharmaceutical Quality System) provide a comprehensive framework emphasizing a quality-by-design (QbD) approach, risk management, and continuous improvement for drug substance and drug product manufacturing processes.
Types of Process Validation

When we talk about process validation, we all have different ideas about how the process typically goes. This is because we have a few options to choose how we validate our processes according to our requirements and the type of processes and facilities we have. You can choose between:
Traditional Process Validation
Traditional process validation is usually carried out after the pharmaceutical and process development stages are complete, following the scale-up to production scale but before the product is marketed. The primary goal is to confirm that manufacturing can reliably produce products that meet predetermined specifications and quality attributes.
Process validation studies may be performed on pilot-scale batches for products not yet scaled to full production levels. These pilot batches should represent at least 10% of the production scale batch size, ensuring that the scale-up factor does not exceed tenfold. This rule ensures that the pilot scale data is relevant and can predict production scale outcomes accurately.
The quantity of batches produced and samples collected should be determined by quality risk management principles, enabling the identification of standard variations and trends and yielding ample data for assessment.
A minimum of three batches is typically required, although fewer batches may be acceptable with proper justification and supporting data from pilot scale batches.
A protocol for process validation should be developed, outlining the essential process parameters (CPPs), critical quality attributes (CQAs), and their respective acceptance criteria, which must be grounded in either development data or established process knowledge. The protocol for process validation must encompass but is not restricted to:
- A brief overview of the process and its link to the Master Batch Record;
- Assigned roles and responsibilities;
- An overview of the CQAs to be examined;
- An overview of CPPs and their specified limits;
- An overview of additional (non-critical) attributes and parameters that will be assessed or monitored during the validation process, along with the justification for their inclusion;
- An overview of the equipment and facilities to be utilized, including the status of their calibration (including measurement, monitoring, and recording devices);
- A listing of analytical methods and, if needed, their validation;
- Suggested in-process controls with their acceptance criteria and the rationale for selecting each in-process control;
- Extra tests to be conducted, along with their acceptance criteria;
- The sampling strategy and the logic behind it;
- Techniques for documenting and analyzing outcomes;
- Procedures for the release and certification of batches, where applicable.
RELATED: Batch Manufacturing Records in GMP
Continuous Process Verification
Continuous Process Verification (CPV) represents an innovative approach to process validation that diverges from traditional methods by emphasizing ongoing monitoring and assessment of a manufacturing process’s performance.
This approach is detailed in ICH Q8 and allows for real-time verification that a process remains within its specified parameters, consistently yielding products that meet their Critical Quality Attributes (CQAs) and adhere to the established control strategy.
Unlike traditional process validation, which often relies on predefined tests and evaluations conducted at specific points in time, CPV involves continuous process monitoring using advanced analytical technologies and methodologies.
This can be done through in-line, online, or at-line controls, ensuring that each product batch meets quality standards. It requires collecting and analyzing data on quality attributes of materials or components, in-process materials, and finished products. This includes verifying specific attributes, parameters, and endpoints and analyzing CQAs and Critical Process Parameters (CPPs) trends.
Process Analytical Technology (PAT) tools, such as Near-Infrared (NIR) spectroscopy, can facilitate CPV by providing real-time data on the process. Multivariate Statistical Process Control (MSPC) is another tool that supports CPV by applying statistical methods to monitor process variability.
Hybrid Approach
A hybrid approach to process validation involves using both traditional process validation and continuous process verification for different steps within the manufacturing process. This approach allows for flexibility and optimization based on each process step’s specific requirements and complexity. The validation requirements, including batch size and number of batches, depend on the extent to which continuous process verification is employed.
Design Space Verification
In some cases, a design space is established during product development. The design space represents the multidimensional combination and interaction of input variables and process parameters that ensure product quality. Design space verification is necessary when there are changes within the design space, such as moving from one area to another or changing the target operating range.
Design space verification involves confirming the suitability of the design space and ensuring that all critical quality attributes are met in the new area of operation. The verification may include additional testing and controls to assess quality attributes and process parameters. The extent of design space verification depends on the change and the level of risk associated with it.
Concurrent Process Validation
In some situations, when the advantages for the patient significantly outweigh the risks, it might be permissible to begin regular production without finishing the usual validation process, opting instead for concurrent validation. This approach, however, needs to be well-reasoned, clearly recorded in the Validation Master Plan (VMP) for transparency, and received approval from authorized officials.
When opting for concurrent validation, gathering data to prove that each product batch is consistent and meets all the required standards is essential. The findings and final assessments must be comprehensively documented and reviewed by the Qualified Person before the product batch is approved for use.
Continued Process Verification (On-going Process Verification)
“Continued process verification,” as outlined by the FDA, constitutes a segment of process validation, notably the final phase, succeeding the initial stage of “design and development” and the second stage of “process qualification.”
This phase is dedicated to ensuring the process remains under consistent control. It encompasses regularly monitoring process parameters, data trending, managing changes, retraining, and implementing corrective and preventive actions (CAPA).
Scale-up Considerations
It is crucial to gather information through well-designed development and process optimization studies during the scale-up process, from laboratory to pilot to production scale. This information ensures that scale-up can be achieved without compromising product quality.
Variations in batch size should be justified to ensure that they do not adversely affect the critical quality attributes of the finished product. Parameters listed in the process validation scheme should be re-validated when further scale-up is proposed unless the process has been proven to be scale-independent or continuous process verification is employed.
Control Strategy

Developing a robust control strategy is important for ensuring the quality and consistency of drug products (DP). This strategy is based on accumulated knowledge and insights from comprehensive product and process studies. When developing the Control Strategy, you should take into consideration the following:
- An initial risk assessment identifying certain material attributes and process parameters as high risk to the Drug Product’s (DP) – Critical Quality Attributes (CQAs)
- Critical Material Attributes (CMAs) and Critical Process Parameters (CPPs) were established.
- The Operating Ranges were pinpointed.
- All variables considered high risk were incorporated into the control strategy.
10 Process Validation Tips
- Adopt an ongoing approach to monitoring and improving processes, maintaining quality and efficiency rather than relying solely on periodic checks.
- Identify, understand, and mitigate process variation to ensure consistent output quality, recognizing that controlling variability is fundamental to process predictability and product reliability.
- Establish critical process variables directly from the design phase, enabling precise control over those factors that influence the process’s outcome from the outset.
- Guarantee process reliability and product quality by integrating control measures into the process design rather than depending on post-production testing to identify and correct flaws.
- Acknowledge and build upon the quality control measures and process optimizations already implemented within the company, formalizing and enhancing existing practices for improved outcomes.
- Demonstrate through documented evidence how implemented control strategies effectively maintain the process within defined limits, ensuring transparency and accountability.
- Invest in a thorough analysis and understanding of the process to minimize the necessity for extensive corrective actions later, emphasizing the importance of proactive planning in process validation.
- Base all process-related decisions on robust, scientifically valid, and statistically sound data, coupled with a risk management approach, to ensure decisions are objective, defensible, and aligned with best practices.
- Ensure all facilities and equipment are tested and verified to consistently perform to the specified standards, highlighting the necessity of reliable infrastructure in process validation.
- Apply evaluation and control measures to established processes, ensuring they continue to produce quality outputs and are regularly reviewed for potential improvements, highlighting the ongoing nature of process validation even for long-standing operations.
Revalidation
Re-validation is the process of repeating process validation to ensure that any changes made in the process or equipment, as per change control procedures, do not negatively impact the process characteristics and product quality. This is crucial because changes can potentially alter the product’s efficacy, safety, and quality.
The specific changes that often necessitate revalidation include:
- Raw Materials: Changes in the physical properties of raw materials (like density, viscosity, particle size) that could affect the process or product.
- Starting Material Manufacturer: Changing the manufacturer of starting materials can impact the consistency and quality of the final product.
- Packaging Material: Substituting materials used for packaging (e.g., from plastic to glass) could affect product stability and quality.
- Process Changes: Modifications in the manufacturing process, such as mixing times and drying temperatures.
- Equipment Changes: Introduction of new equipment or significant modifications to existing equipment.
- Production Area and Support System Changes: Re-arrangements of production areas or changes in support systems like a new water treatment method.
- Transfer of Process: Moving the process to another site can affect the product’s consistency and quality.
- Unexpected Changes: Those found during self-inspection or routine analysis of process trend data.
FAQ
How Does Risk Management Relate to Process Validation?
Risk management is integral to process validation, identifying potential failure points in the process and implementing controls to mitigate these risks, ensuring the process remains in control and produces quality products.
What Are Some Common Methods Used in Process Validation?
Common methods include statistical process control, design of experiments, and failure mode and effects analysis (FMEA) to assess and control variability.
What Is the Purpose of Using Data Validation During Your Analysis Process?
The purpose of using data validation during the analysis process is to ensure the accuracy, completeness, and reliability of the data before it is used for decision-making or further analysis, thereby minimizing errors, improving the quality of insights derived from the data, and supporting the integrity of conclusions drawn from the analysis.
Why Are 3X Sampling Plans Implemented in Process Validation?
3X sampling plans are implemented in process validation to ensure high confidence in the process capability and product quality. Testing the process at three times the normal production scale or frequency provides comprehensive data across a range of operating conditions, thereby identifying potential variability and ensuring that the process is stable, capable, and consistently producing products that meet predefined quality specifications.
Conclusion
Process Validation in GMP is critical to ensuring the safety, efficacy, and quality of pharmaceutical products. It involves a series of activities designed to demonstrate that the manufacturing processes consistently produce products that meet predefined quality standards.
By following the key principles, conducting validation activities, and implementing best practices, pharmaceutical companies can ensure compliance with regulatory requirements and maintain the integrity of their manufacturing processes.














