Table of Contents
Process Validation is a rigorous, data-driven approach that seeks to establish, through concrete evidence, that a manufacturing process can consistently deliver products that meet predetermined specifications.
This article aims to show the importance of the Process Validation Lifecycle, exploring its stages, challenges, and the critical role it plays in maintaining product integrity from development to commercialization.
The Essence of Process Validation
Process Validation is a critical and systematic approach employed to confirm that a process consistently produces a result or product meeting its predetermined specifications and quality attributes. This concept is paramount in industries where safety and efficacy are paramount, such as pharmaceuticals, biotechnology, and medical devices.
The essence of process validation lies in its ability to establish confidence through scientifically collected and analyzed data, proving that a process, when operated within specified parameters, can consistently deliver products that meet all quality requirements.
Furthermore, process validation helps organizations comply with regulatory requirements. Regulatory bodies, such as the Food and Drug Administration (FDA), require companies to demonstrate that their manufacturing processes are capable of consistently producing products that meet the desired specifications.
Read more about the importance of process validation in our article.
Process Validation Lifecycle – Overview
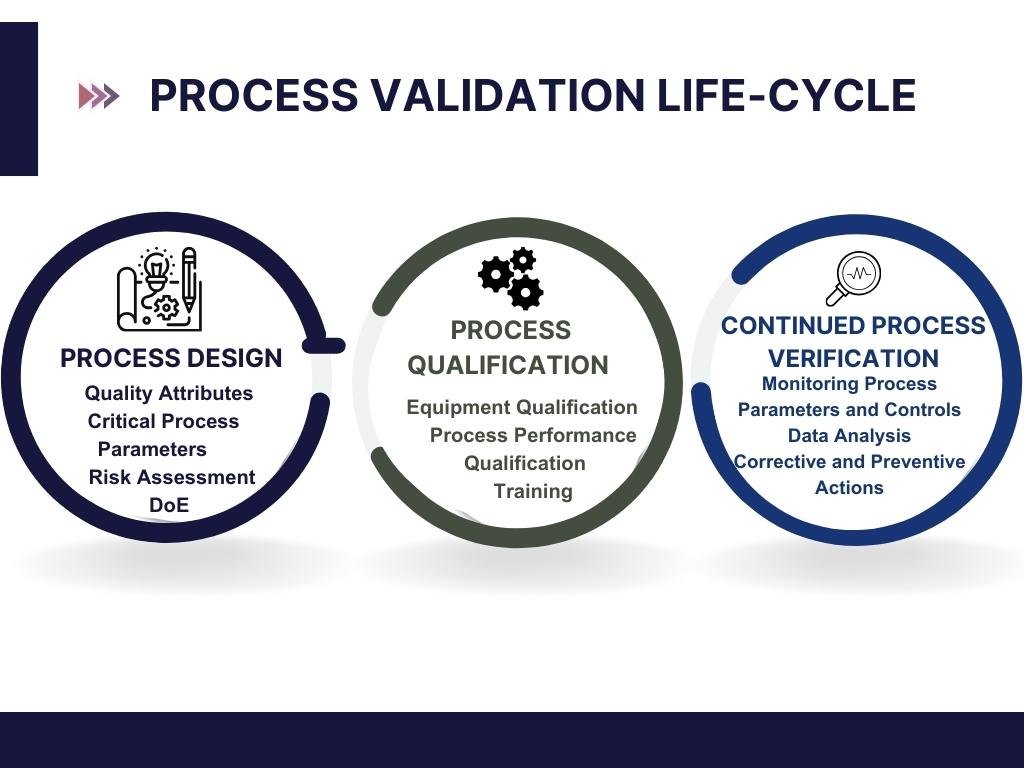
The core objective of process validation is to ensure product quality, safety, and efficacy. It involves a series of activities taking place over the lifecycle of the product and the process. This lifecycle approach is divided into three main stages: Process Design, Process Qualification, and Continued Process Verification.
1. Process Design: The first stage involves understanding the process and defining the commercial manufacturing process based on knowledge gained through development and scale-up activities.
- Creation of a Quality Target Product Profile (QTPP).
- Identifying Critical Quality Attributes (CQAs).
- Defining Critical Process Parameters (CPPs).
- Conducting risk assessments to ensure product quality and safety.
2. Process Qualification: During this stage, the process design is evaluated to determine if the process is capable of reproducible commercial manufacturing. This involves both the qualification of the equipment and facilities used (Installation Qualification, Operational Qualification) and the process performance qualification.
- Evaluation of buildings and facilities to ensure compliance with local and pharmaceutical manufacturing regulations.
- Verification of the transportation and storage of raw materials.
- Assessment of production line employees’ knowledge, training, and working practices.
- Detailed monitoring of every step of the manufacturing process, including predefined sampling points.
- Ensuring the finished product’s packaging, storage, and distribution meet quality standards.
3. Continued Process Verification: This ongoing stage ensures the process remains in a state of control during commercial production, adjusting for variability in materials, equipment, and the process itself.
- Ensure the process remains in control during commercial production.
- Monitor and control process parameters and quality attributes.
- Collect and analyze data to identify trends and variability.
- Implement adjustments to maintain product quality.
- Continually ensure the product meets quality requirements through ongoing control and adjustments.
Stage 1 – Process Design
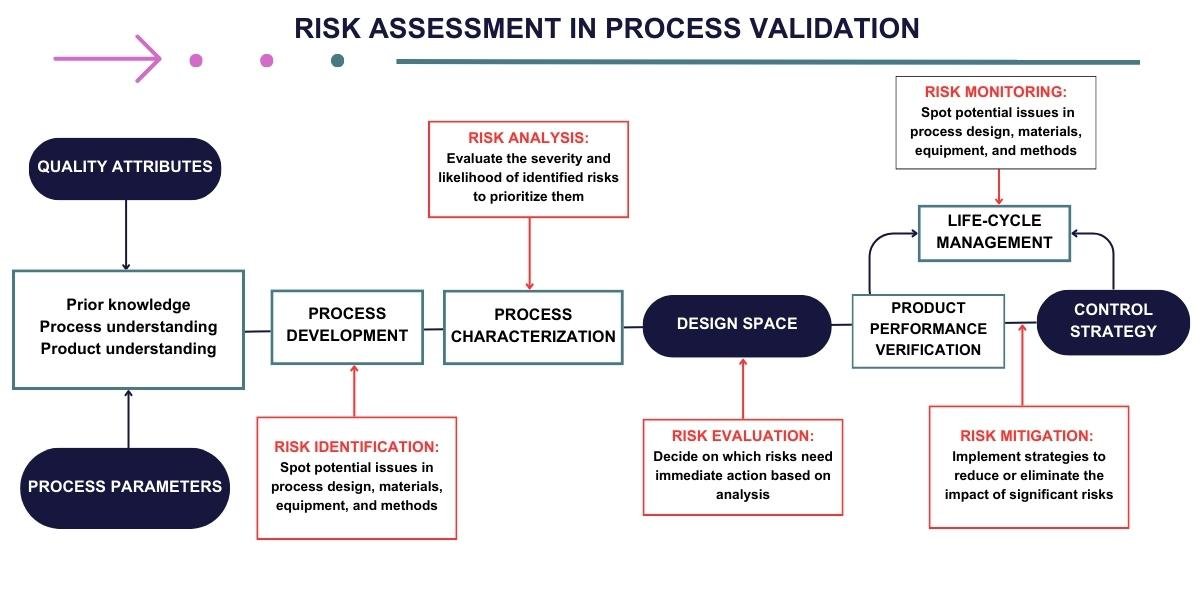
The manufacturing process is developed and designed in this stage based on scientific principles and quality risk management. It involves identifying critical process parameters and their acceptable ranges and establishing control strategies.
Building and Capturing Process Knowledge and Understanding
At the initial stages of process design in pharmaceutical development, it is not required to adhere to the cGMP conditions that are required for later stages, such as process qualification (Stage 2) and continued process verification (Stage 3). While exempt from cGMP, these early experiments should still follow scientific methods and principles to ensure reliability and validity. Good documentation practices are emphasized to record and track the experiments and their outcomes accurately.
The guidance for conducting early process design experiments in a scientifically sound manner aligns with the ICH Q10 Pharmaceutical Quality System, which outlines a comprehensive model for quality management throughout the lifecycle of a pharmaceutical product.
The information gathered during product development, including the dosage form, product quality attributes, and an overview of the manufacturing process, is crucial for the process design stage.
When designing the process, it’s important to consider the functionality and limitations of commercial-scale manufacturing equipment and factors that could introduce variability into the production process. These include differences in raw material lots, operator performance, environmental conditions, and measurement systems.
Laboratory or pilot-scale models can be employed to anticipate variability in the commercial manufacturing process. These models help estimate and understand the potential variability in the production process.
DOE (Design of Experiment) studies help understand the process by identifying how different variables affect the process outcomes. These studies can reveal complex interactions between variables and help optimize the process design.
SEE ALSO: Quality by Design in Pharmaceutical Development
Establishing a Strategy for Process Control
The main goal of process control strategies is to manage and reduce variability in the manufacturing process. This variability can come from different sources (like raw materials or the manufacturing process itself), and it can negatively affect the quality of the final product. Process controls are implemented to ensure the product meets the desired quality standards.
Types of Process Controls:
- Reducing Input Variation: This strategy involves decreasing the variability of input materials or conditions before the manufacturing process.
- Adjusting for Input Variation: This approach accepts that there will be some variation in inputs and focuses on adjusting the manufacturing process in real time to compensate for this variation, thus minimizing its impact on the final product.
- Combination of Both: Often, the most effective process control strategies use a mix of both approaches to tackle input variation from multiple angles.
More sophisticated process control strategies might employ Process Analytical Technology (PAT), which allows for the real-time analysis and adjustment of processing conditions. Using PAT, manufacturers can ensure that despite variations in inputs or process conditions, the output remains consistent in quality.
Stage 2 – Process Qualification
Process Qualification (PQ) is an essential stage within the broader framework of process validation, which is a critical aspect of quality assurance in cGMP.
Process validation ensures that manufacturing processes can consistently produce a product that meets predetermined quality criteria. The PQ stage, in particular, focuses on evaluating and verifying that the process design is theoretically sound and practically capable of reproducible commercial manufacturing. This evaluation is split into two main elements: the facility’s design and the equipment and utilities qualification and process performance qualification (PPQ).
Successful completion of Stage 2 is a prerequisite for moving forward to commercial distribution. If they meet the established quality criteria, products manufactured during this stage can be released for distribution. This allows for the transition from validation to actual production, where the processes and systems validated during PQ are put into regular operation, with ongoing quality monitoring to ensure continued compliance with all necessary standards and regulations.
Design of a Facility and Qualification of Utilities and Equipment
Proper design of the manufacturing facility is mandated to ensure that the environment is conducive to producing high-quality products. This includes considerations for layout, material flow, and environmental conditions (e.g., temperature, humidity) to minimize the risk of contamination, cross-contamination, and errors.
Before proceeding to Process Performance Qualification (PPQ), the facility and its systems (utilities, equipment) must be commissioned and qualified. This means ensuring they are correctly installed, operate as intended, and suit their intended use.
The Quality Control (QC) or Quality Assurance (QA) unit within the organization plays a crucial role in reviewing and approving both the qualification plan and the final qualification report. This ensures that all qualification activities meet the established standards and regulatory requirements.
Process Performance Qualification
Process Performance Qualification (PPQ) is a blend of various elements—qualified facilities, utilities, equipment, and trained personnel—alongside the actual manufacturing process, control procedures, and components to produce commercial batches of a product.
PPQ integrates all critical components—qualified facilities, utilities, equipment, and trained personnel—with the commercial manufacturing process. This integration aims to produce commercial batches of a product, ensuring that the entire system functions cohesively and effectively at a commercial scale.
Successful completion of the PPQ is mandatory before a manufacturer can begin distributing the drug product commercially. This requirement ensures that the product manufactured at a commercial scale meets all quality standards and regulations.
The approach to conducting a PPQ is grounded in solid scientific principles. It requires a comprehensive understanding of the product and process, ensuring that every aspect of the manufacturing is under control.
The conditions under which the PPQ is conducted are based on cumulative data from various studies, including designed experiments and batches produced at different scales. This data helps in fine-tuning the manufacturing conditions to optimize product quality.
Factors such as production volume, process complexity, and understanding of the process influence the duration of enhanced monitoring and testing. Experience with similar products and processes also plays a crucial role in determining this period.
The ultimate objective of PPQ and process validation is to establish scientific evidence that the manufacturing process is reproducible and can consistently produce quality products. This evidence forms the basis for confidence in the process’s capability to meet quality standards on an ongoing basis.
The goal of validating any manufacturing process is to establish scientific evidence that the process is reproducible and will consistently deliver quality products.
PPQ Protocol
The PPQ (Process Performance Qualification) protocol is a detailed document crucial for the second stage of process validation in pharmaceutical manufacturing. This protocol ensures that the manufacturing process is designed correctly and can consistently produce high-quality products under commercial production conditions.
Some of the key elements that should be included in the PPQ protocol are:
1. Manufacturing Conditions
- Operating Parameters and Processing Limits (conditions under which the manufacturing process operates, including temperatures, pressures, times, and other critical parameters that influence product quality)
- Component Inputs (raw materials used, including specifications and handling requirements).
2. Data Collection and Evaluation
This includes process monitoring data, quality control results, and any other relevant information that can influence the assessment of the process’s capability.
3. Testing and Acceptance Criteria
- Tests to be Performed (in-process, release, and characterization tests)
- Acceptance Criteria
4. Sampling Plan
Detailed strategy for taking samples throughout the manufacturing process, specifying sampling points, the number of samples, and the sampling frequency.
5. Criteria and Process Performance Indicators
Establishes the benchmarks and performance indicators used to make a science- and risk-based decision on the process’s capability to produce quality products consistently. This section should include:
- Statistical Methods – Description of the statistical analyses to be applied to the collected data, focusing on intra-batch and inter-batch variability
- Deviation Management
6. Facility and Equipment Design
If not already addressed in previous stages, the protocol should cover the design of the manufacturing facilities, the qualification of utilities and equipment, and personnel training. Verification of material sources, including components and container/closures, should also be included to ensure all elements meet quality standards.
7. Validation of Analytical Methods
Confirms the status of analytical method validation, ensuring that the methods used for process monitoring, in-process materials, and the final product are accurate, reliable, and validated.
8. Review and Approval
The protocol requires a thorough review and approval by relevant departments, including the quality unit, to ensure it meets all regulatory standards and internal quality assurance requirements.
PPQ Protocol Execution and Report
The execution and reporting of the Process Performance Qualification (PPQ) protocol are vital steps in the process validation framework. These steps ensure the manufacturing process can consistently produce products that meet quality specifications and regulatory standards.
PPQ Protocol Execution
- Review and Approval: Before starting the PPQ protocol execution, it must be thoroughly reviewed and approved by all relevant departments, including the quality unit, to ensure it meets all regulatory requirements and internal standards.
- Adherence to Established Procedures: Any deviations from the established protocol must follow predefined procedures or allowances within the protocol itself.
- Commercial Manufacturing Conditions: The PPQ protocol must be executed under the commercial manufacturing process and routine procedures.
- Normal Operating Conditions: The execution should involve the personnel who routinely perform the manufacturing process using the standard utility systems, materials, environment, and procedures.
PPQ Report Preparation
After completing the PPQ protocol execution, a comprehensive report is prepared, covering several key areas:
- Protocol Adherence: The report discusses and references all aspects of the PPQ protocol, ensuring that the execution was aligned with the planned activities and conditions.
- Data Summary and Analysis: It summarizes and analyzes the data collected during the PPQ execution, as specified by the protocol. This analysis is crucial for understanding the process’s performance and identifying any areas of concern.
- Unexpected Observations: The report evaluates any unexpected observations and additional data not specified in the protocol.
- Manufacturing Nonconformances: Any deviations, unexpected test results, or other relevant information affecting the process’s validity are summarized and discussed. This includes a detailed description of any corrective actions or changes recommended for existing procedures and controls.
- Conclusions and Justifications: A clear conclusion is provided, stating whether the process met the conditions established in the protocol and is considered to be in control. If the conclusion is negative, the report specifies what needs to be addressed before reaching a positive conclusion.
- Review and Approval: The report includes reviews and approvals from all appropriate departments and the quality unit, ensuring it meets the required standards and regulatory compliance.
The execution and reporting of the PPQ protocol are critical for confirming that a pharmaceutical manufacturing process is under control and capable of consistently producing products that meet all quality requirements. This process validates the manufacturing process and provides a documented basis for making informed decisions about the process’s readiness for commercial production.
Stage 3 – Continued Process Verification
The final stage of process validation is continued process verification, which ensures that the process remains controlled during commercial manufacturing. It involves implementing a system or systems to detect deviations from the process as designed and monitoring process performance to identify variability and opportunities for improvement.
Data should be collected and analyzed during continued verification to evaluate process stability and capability. The data should include trends in process parameters and quality attributes and be statistically trended and reviewed by trained personnel. The quality unit should periodically review the data and assess process stability and capability. Any deviations, out-of-specification results, or process yield variations should be investigated, and corrective actions should be taken as necessary.
The continued process verification program should include monitoring and sampling process parameters and quality attributes to ensure the process remains in control. The monitoring and sampling should be adjusted based on the variability estimates obtained during the process qualification stage. Any proposed changes to the process should be documented, justified, and approved by the quality unit before implementation.
Risk Assessment in the Process Validation Lifecycle
Risk assessment is integral to the Process Validation Lifecycle, acting as a strategic tool to ensure that manufacturing processes consistently produce products that meet predefined quality and safety standards. Within this lifecycle, risk assessment helps identify, analyze, and mitigate potential process-related risks, ensuring the reliability and efficacy of the end product.
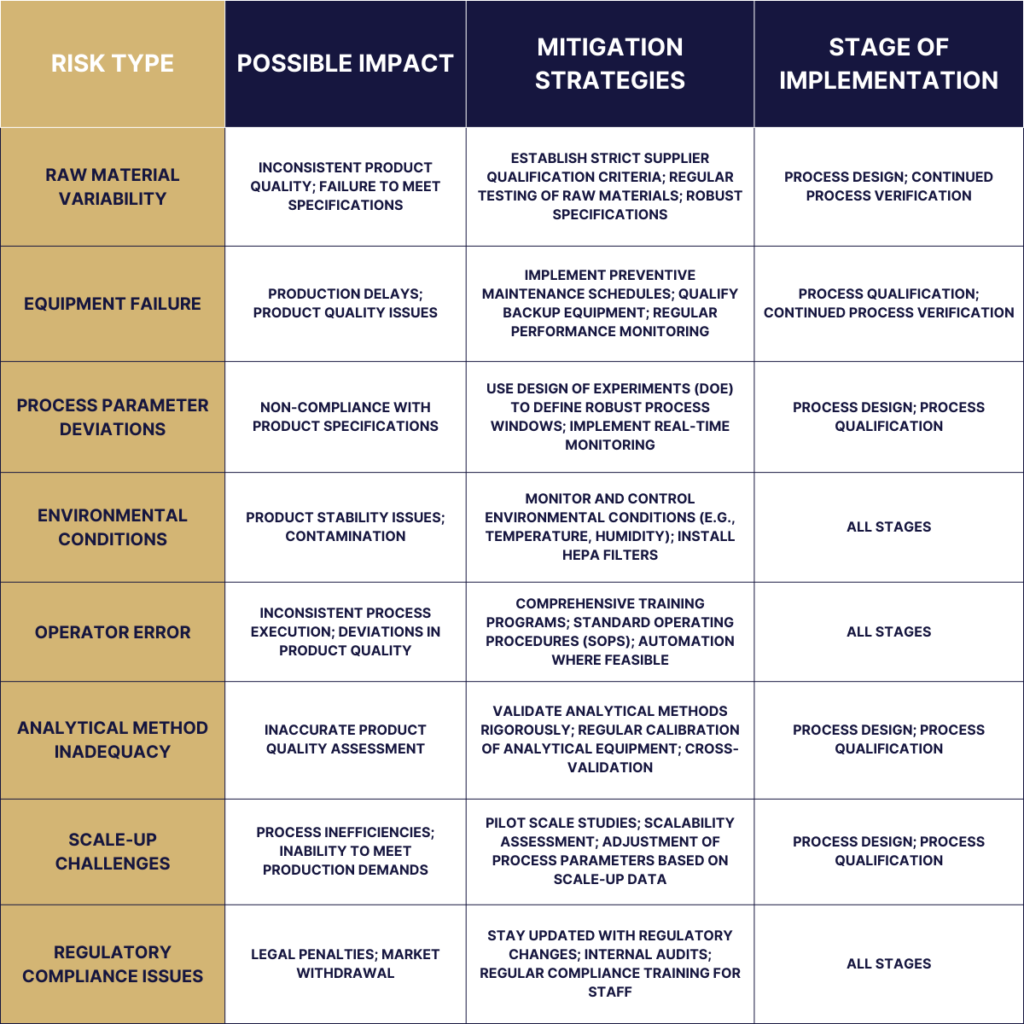
Here’s how risk assessment is applied across the different stages of the Process Validation Lifecycle:
During Process Design
- Identifying Risks: Initial risk identification focuses on the product design and process development stages. Potential risks related to process parameters, material variability, and equipment limitations are identified.
- Analysis and Mitigation: Risks are analyzed for their potential impact on product quality. Strategies are then developed to mitigate these risks, such as optimizing process parameters and establishing robust control systems.
During Process Qualification
- Risk Evaluation in Scale-up: As the process transitions from pilot to commercial scale, risks associated with scale-up, equipment qualification, and process performance are evaluated. This includes assessing the capability of the process to meet quality attributes consistently.
- Implementing Mitigation Strategies: Mitigation strategies may involve adjustments to equipment, process parameters, or the training of personnel to ensure the process remains within defined quality parameters.
During Continued Process Verification:
- Ongoing Risk Monitoring: Continuous monitoring of the process allows for the identification of new risks or changes in previously identified risks. This could be due to changes in raw materials, equipment wear and tear, or variability in environmental conditions.
Review and Adjustment of Mitigation Strategies: Based on ongoing risk assessment, mitigation strategies are reviewed and adjusted as necessary to ensure continuous control over the process and maintenance of product quality.
How Can Risks Be Identified and Managed During the Process Validation Lifecycle?
Risks can be identified and managed through risk assessment tools such as Failure Mode and Effects Analysis (FMEA) and by implementing risk mitigation strategies throughout the validation lifecycle.
How Can Deviations and Non-conformances Be Handled During Process Validation Lifecycle?
Deviations and non-conformances should be documented, investigated, and addressed promptly during process validation lifecycle. Corrective and preventive actions should be implemented to prevent recurrence and ensure process integrity.
How Can Continuous Improvement Be Achieved in the Process Validation Lifecycle?
Continuous improvement in the process validation lifecycle can be achieved through data analysis, trending, and feedback mechanisms. Regular reviews, audits, and training can also help identify areas for enhancement and optimization.
Can Products Be Released for Distribution Before Completing the Process Validation Lifecycle?
Products can only be released after successful Process Qualification, ensuring they meet established quality criteria.
Final Thoughts On Process Validation Life-Cycle
The process validation lifecycle is a critical aspect of ensuring the quality and reliability of manufacturing processes. By systematically validating their processes, organizations can minimize risks, enhance product quality, comply with regulatory requirements, and maintain a competitive edge in the market.
Understanding the importance of process validation, as well as the regulatory requirements and stages involved, is essential for organizations across various industries. By addressing common challenges and implementing best practices, businesses can optimize their process validation programs, improve customer satisfaction, and achieve long-term success in today’s demanding business environment.















