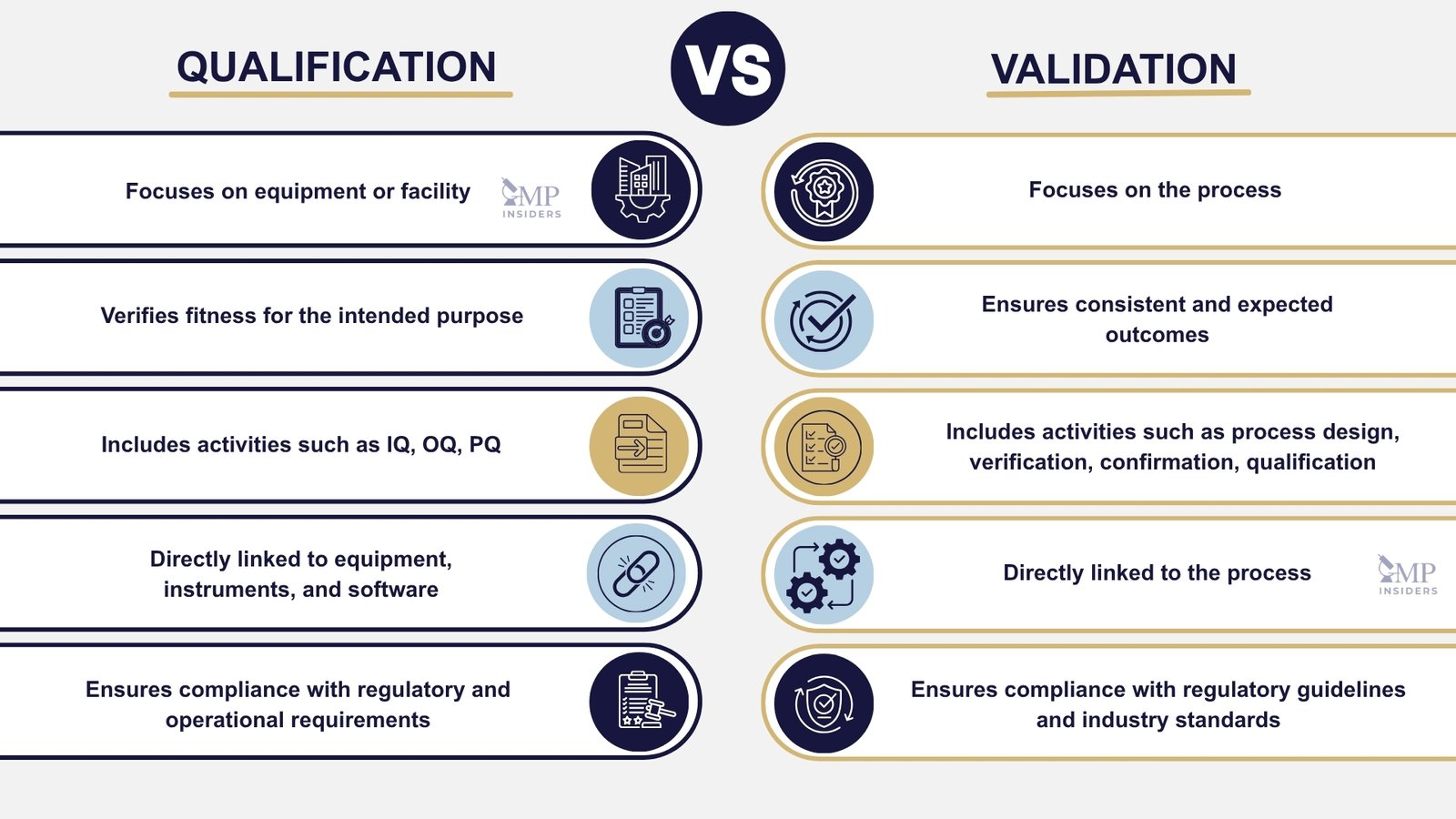
One of the first concepts that regulators expect GMP professionals to fully understand is the difference between qualification and validation, and why confusing the two can lead to major compliance risks.
Regulatory bodies like EMA (EU GMP Annex 15 & Annex 11), FDA (Process Validation Lifecycle Guidance & CSA 2025), WHO, and PIC/S all position qualification as a prerequisite technical demonstration of capability, confirming that equipment, utilities, instruments, and computerized systems are installed and operating exactly as intended.
Once the foundational infrastructure is successfully qualified, validation is then conducted to prove that the process, under real-world variability and worst-case conditions, will consistently deliver compliant results throughout its lifecycle.
This article clarifies the distinction between qualification and validation from a GMP perspective, explains how they are applied across different systems and processes, and outlines their specific regulatory expectations within the pharmaceutical lifecycle.
Qualification: Fitness for Intended Purpose
Qualification is the process of verifying that a piece of equipment or a particular item is fit for its intended purpose. It focuses on ensuring that all aspects of the equipment or facility meet regulatory and operational requirements.
Qualification ensures that the equipment, instruments, and software used in the pharmaceutical industry are functioning properly and capable of producing consistent results. This process is directly linked to the equipment rather than the overall process.
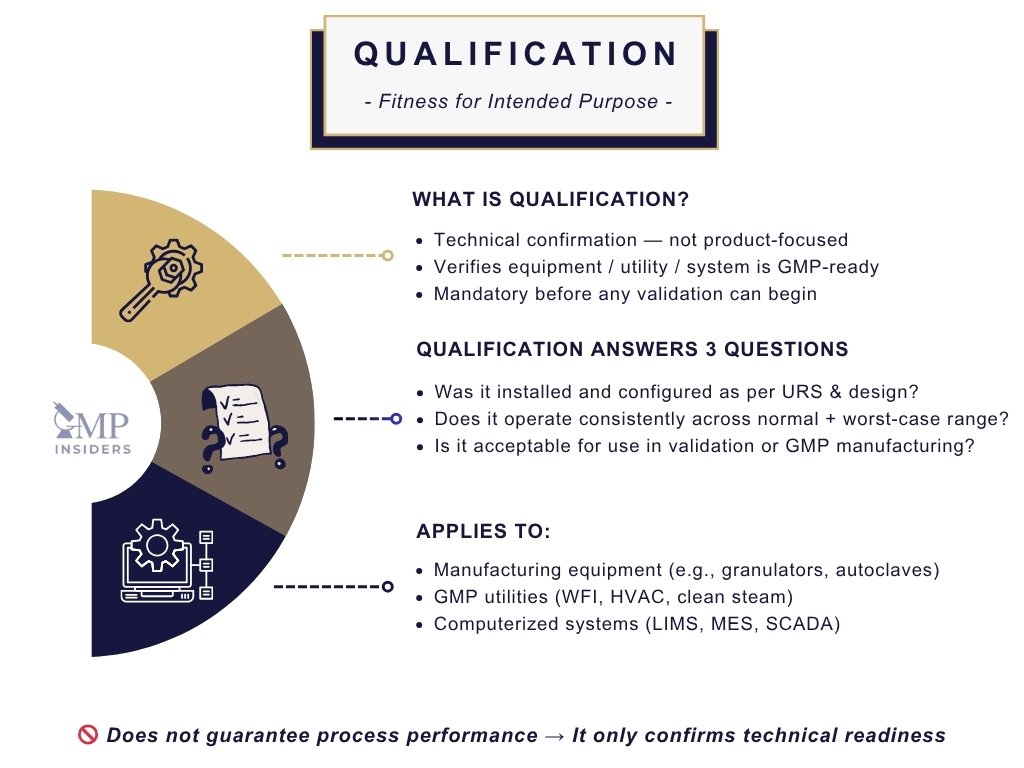
Regulatory guidance, such as EU GMP Annex 15, clearly defines qualification as a prerequisite stage that must be completed before process validation. The FDA Process Validation Guidance also considers qualification to be an integral part of the validation lifecycle. If a system is not qualified, any subsequent validation is considered invalid from a regulatory standpoint.
Qualification is not yet focused on product quality outcomes. Its purpose is to confirm technical readiness. In practice, it answers three essential questions:
- Has the system been installed and configured in accordance with the approved design and user requirements?
- Does it operate consistently across its defined operating range, including worst-case conditions?
- Is it acceptable to release the system for use in validation or GMP manufacturing activities?
This applies to critical equipment, manufacturing utilities such as WFI and HVAC, and computerized systems such as LIMS, MES, and SCADA, where Annex 11 and FDA 21 CFR Part 11 expectations regarding data integrity and access control already apply at the qualification stage.
Qualification does not guarantee process performance. It only confirms technical suitability, a fundamental regulatory gate before validation can begin.
Qualification Step Structure
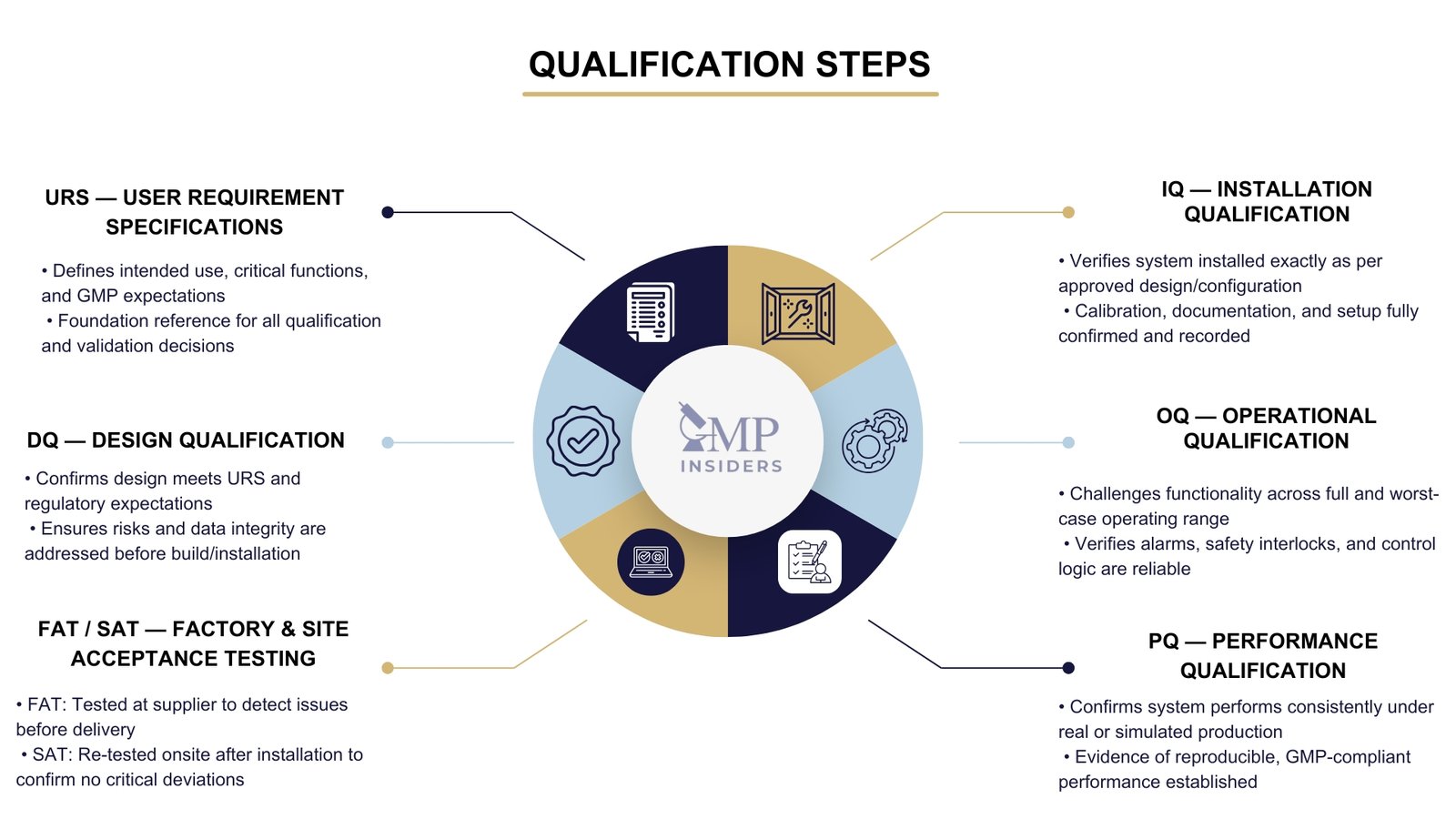
Qualification follows a structured and traceable sequence aligned with the validation lifecycle. The process ensures that each stage builds on documented and approved decisions, with full traceability back to the User Requirements Specification (URS).
User Requirements Specification (URS)
The foundation of the qualification process lies in the User Requirements Specification (URS). In this stage, the specifications for equipment, facilities, utilities, or systems are defined. The URS serves as a reference point throughout the validation life cycle. Essential quality elements must be incorporated at this stage, and any GMP risks should be mitigated to an acceptable level.
SEE ALSO: How to Write a User Requirement Specification
Design Qualification (DQ)
DQ verifies and documents that the proposed design meets the URS and regulatory expectations. At this stage, GMP risks must be identified, controlled, and justified. For computerized systems, Annex 11 and data integrity elements must be considered from the start.
FAT / SAT (Factory and Site Acceptance Testing)
- FAT is performed at the supplier’s facility, primarily for complex systems or equipment. It allows early issue detection before delivery.
- SAT is performed after delivery and installation on site. It confirms that no critical functional elements were affected by transport, installation, or integration into existing systems.
SEE ALSO: FAT and SAT in GMP
Installation Qualification (IQ)
IQ documents that the system is installed exactly as specified in the approved design. This includes verification of mechanical installation, connections, calibration status, spare parts lists, manuals, software versions, system configuration, and as-built documentation.
| Verification Aspect | Criteria |
|---|---|
| Correct installation of components | Verified against engineering drawings |
| Calibration of instrumentation | Completed |
| Materials of construction | Verified |
| Supplier operating and maintenance instructions | Collected and collated |
Operational Qualification (OQ)
OQ confirms that the system operates as intended across its defined operating ranges, including upper and lower limits and relevant worst-case conditions. Alarm behavior, interlocks, and safety functions are typically challenged at this stage.
| OQ Aspect | Criteria |
|---|---|
| Tests for system operation as designed | Passed |
| Confirmation of upper and lower operating limits | Verified |
Successful completion of OQ allows for the finalization of standard operating and cleaning procedures, operator training, and preventative maintenance requirements.
Performance Qualification (PQ)
PQ demonstrates that the system consistently performs under real or simulated production conditions using qualified materials or equivalent substitutes. For equipment used in sterile or high-risk operations, this includes testing under actual batch conditions and verifying that the system supports compliant and reproducible manufacturing outcomes.
While PQ often occurs separately, it may be appropriate to perform it in conjunction with OQ or Process Validation in some cases.
| PQ Aspect | Testing Criteria |
|---|---|
| Tests using production materials or equivalents | Performed |
| Frequency of sampling justified | Verified |
| Operating range coverage (unless documented evidence is available) | Verified |
Application of Qualification in GMP
Qualification is performed on all critical physical and technical elements that support GMP manufacturing and quality control. Typical qualification targets include:
- Manufacturing equipment — to confirm that machines such as granulators, sterilizers, blister lines, or filling units function according to approved operating specifications.
- Support and environmental systems — such as HVAC, compressed air, cleanroom monitoring, and automation layers that indirectly affect product quality and aseptic control.
- Laboratory and in-process control instruments — ensuring analytical devices (HPLC, balances, TOC analyzers, etc.) deliver accurate and reproducible measurements.
- GMP utilities — including purified water, WFI, clean steam, nitrogen, and other critical utility systems that must consistently meet defined quality attributes.
Qualification is required wherever equipment, utilities, or controlled environments have a direct or indirect impact on product quality, patient safety, or GMP data integrity.
Validation: Proven Consistency Over Time
Validation is the documented process of demonstrating that a GMP-relevant process, cleaning procedure, analytical method, or computerized system consistently delivers results that meet predefined acceptance criteria under routine operating conditions.
Unlike qualification, which confirms technical fitness, validation demonstrates consistent, reproducible performance over time, with a direct impact on product quality, patient safety, and regulatory approval.
Validation is defined by EU GMP Annex 15, the FDA Process Validation Lifecycle Guidance, ICH Q2 (analytical methods), Annex 11 / FDA 21 CFR Part 11 (computerized systems) and is maintained through lifecycle control, not as a one-time event. It begins only after the supporting equipment and systems have first been qualified.
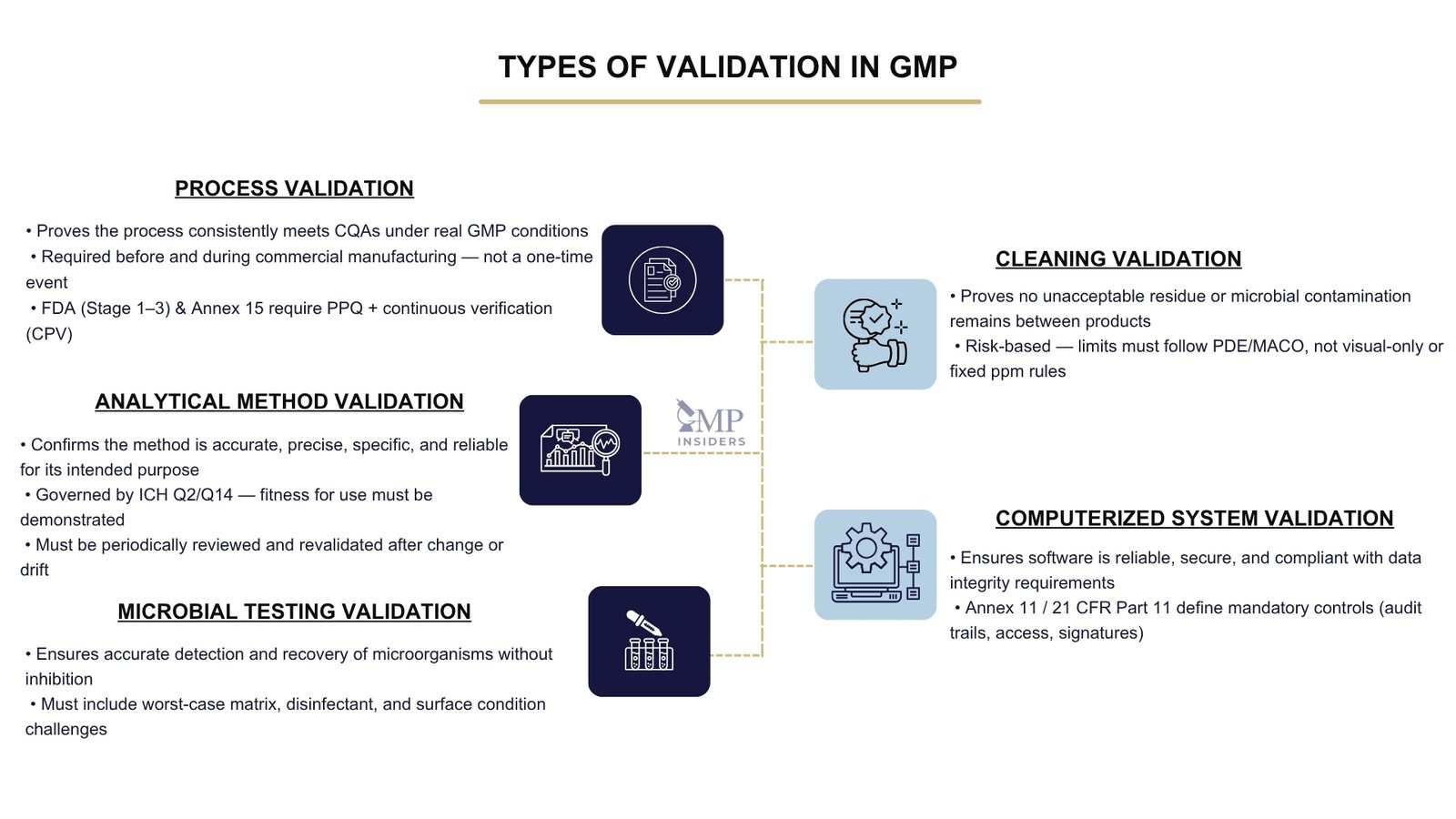
Process Validation
Process validation is the primary mechanism for demonstrating that the manufacturing process can reliably deliver a product that meets its critical quality attributes (CQAs). This applies during initial product introduction, post-change implementation, and throughout continued commercial supply.
Regulators expect alignment with either:
- FDA’s 3-Stage Process Validation Lifecycle
• Stage 1: Process Design
• Stage 2: Process Performance Qualification (PPQ)
• Stage 3: Continued Process Verification (CPV) - EU GMP Annex 15 approach, where PPQ is followed by ongoing performance monitoring and control strategy maintenance.
SEE ALSO: Process Validation Lifecycle: Risk-Based Approach
Validation Approaches
The approach selected for validation depends on factors such as product lifecycle stage, process understanding, regulatory expectation, and quality risk assessment. Regulatory agencies do not enforce one single method, they expect manufacturers to justify their chosen approach scientifically and risk-based.
| Approach | When It’s Used | Key Advantage | Regulatory Expectation | Typical Risk |
|---|---|---|---|---|
| Traditional | New product introduction | Proven, widely accepted | 3 batches, protocol-driven | Slow, high cost |
| Concurrent | Medical urgency | No supply delay | Requires pre-approval, full batch hold | High regulatory scrutiny |
| Continued Process Verification (CPV) | Mature / QbD-based processes | Real-time lifecycle monitoring | Must show statistical control | Requires automation / data integrity systems |
| Hybrid | Scale-up or tech transfer | Balanced flexibility | Justification required | Risk of misalignment |
Traditional (Prospective) Validation
This is the most widely used and historically accepted approach, where validation is performed before routine commercial production begins.
- Typically involves three consecutive full-scale batches manufactured under normal operating conditions.
- The purpose is to demonstrate reproducibility and process robustness before market release.
- This approach remains standard for new product introductions and regulatory filings, primarily when limited prior process knowledge exists.
Concurrent Validation
Used only in exceptional and justified cases, where validation is executed while commercial production is ongoing, meaning patient supply cannot be delayed, but control strategy and risk assessment prove it is safe to proceed.
- Must be pre-approved in the Validation Master Plan (VMP) and documented as a conscious regulatory decision.
- All batches produced remain on hold until successful validation is confirmed.
- Commonly applied in critical market supply or medically necessary situations, not as a convenience method.
Continuous / Ongoing Process Verification (CPV)
This is the most modern, lifecycle-based approach, aligned with FDA Stage 3 and Annex 15 expectation for continual verification.
- Applied especially in Quality by Design (QbD) environments, where the process is fully characterized and statistically controlled.
- Instead of validating a fixed number of batches, real-time monitoring and trending are continuously used as the proof of a validated state.
- Strong use of PAT, statistical process control, and automated data systems is expected.
Hybrid Approach
Combines elements of both traditional validation and ongoing verification.
- Often applied post-change, during scale-up, or when the process has some historical validation data but still requires enhanced monitoring.
- Intended to bridge established process knowledge with modern risk-based control philosophies.
- Frequently used in regulatory requalification or technology transfer scenarios.
Analytical Method Validation
Analytical method validation is the documented process of proving that a test method is scientifically reliable, accurate, and suitable for its intended use in GMP decision-making, whether for raw materials, in-process control, finished product release, stability studies, or cleaning verification.
Regulatory expectations are defined in ICH Q2(R1) and reinforced in EU GMP Annex 15 and FDA Data Integrity guidance, emphasizing fitness for intended purpose and long-term reliability, not just initial validation.
A validated analytical method must demonstrate certain performance characteristics:
- Accuracy — the closeness of test results to a true or accepted reference value
- Precision — including repeatability (same analyst, same conditions) and intermediate precision (different days / analysts / instruments)
- Specificity — ability to distinguish the target analyte from impurities, excipients, degradants, or matrix effects
- Detection Limit (LOD) & Quantitation Limit (LOQ) — essential for trace-level testing and cleaning validation scenarios
- Linearity & Range — proof the method gives valid results across the intended working concentration interval
- Robustness — resilience against small, deliberate parameter variations (e.g., pH, temperature, flow rate, injection volume)
Validation must be completed before the method is used to make any GMP-quality decision, and must be protocol-driven, approved by QA, and supported with full raw data traceability.
For pharmacopoeial (compendial) methods, full validation is not always required, however, method verification is mandatory to demonstrate suitability under the specific laboratory conditions, in alignment with Annex 15 and ICH Q2.
Related Article: Analytical Method Validation vs Verification
Analytical methods are not static, laboratories are expected to periodically review and maintain validation status throughout the lifecycle, evaluating revalidation needs following method changes, instrument upgrades, regulatory updates, or confirmed performance drift.
Microbial Testing Validation
Microbial testing validation ensures that the method can accurately detect, recover, and quantify microorganisms without interference from the product, the testing environment, or the cleaning/disinfection agents in use.
It is critical for both product quality testing and environmental/cleanroom monitoring, where false-negative results could lead to undetected contamination and serious patient risk.
This type of validation is governed by USP <61>, <62>, <71>, Ph. Eur. 2.6.1, 2.6.12, 2.6.13, and supported by EU GMP Annex 1, Annex 15, and WHO guidance.
Microbial testing validation is typically divided into two major applications:

Product-Related Microbial Testing (Method Suitability / Recovery Studies)
This validation confirms that the composition of the product itself does not inhibit microbial recovery. Formulation components such as preservatives, surfactants, or viscosity modifiers can suppress microbial growth and lead to false-negative sterility or bioburden test results.
Validation must demonstrate:
- Valid recovery of compendial test organisms (e.g., S. aureus, P. aeruginosa, C. albicans, A. brasiliensis)
- Neutralization or dilution strategy is effective
- No inhibition from product matrix under the intended test conditions
- Method is suitable before use in routine batch release or stability testing
Cleanroom and Environmental Monitoring Method Validation
Here, the objective is to validate that sampling and detection methods used in cleanrooms reliably recover microorganisms present on surfaces or in air, and that disinfectants do not interfere with microbial recovery.
Focus areas include:
- Agar contact plate or swab method performance
- Recovery at different surface conditions and material types
- Impact of disinfectant residues — must not suppress microbial detection
- Demonstration that the method can detect low-level contamination, especially in Grade A/B zones
Microbial method validation must not be treated as a one-time activity. It requires ongoing performance verification, especially after changes in disinfectants, sampling materials, facility design, or cleaning strategy.
Related Article: Qualification and Validation of Cleanrooms in GMP
Cleaning Validation
Cleaning validation is the documented process of demonstrating that cleaning procedures effectively and reproducibly remove product residues, cleaning agents, and microbial contaminants to a level that is safe for the next batch, especially when equipment is used for multi-product manufacturing.
It is one of the highest-risk areas in GMP, because undeclared cross-contamination is a direct patient safety hazard, and regulators, especially the FDA, EMA, and PIC/S, have repeatedly issued 483s and warning letters when cleaning validation is inadequately justified, not risk-based, or based only on unreliable visual inspection.
Cleaning validation is required to ensure:
- No active pharmaceutical ingredient (API) carryover to the next product
- No residual detergents or cleaning agents remain at harmful levels
- No microbiological contamination persists on equipment surfaces
- All product contact surfaces are validated, including hard-to-clean areas, transfer lines, dead legs, filtration housings, etc.
Regulatory expectations include:
- Toxicologically justified residue limits (e.g., PDE or ADE-based MACO — NOT arbitrary 10 ppm rules anymore — ref: EMA Q&A on PDE, PIC/S PI 046)
- Worst-case selection for cleaning validation (lowest solubility, highest toxicity, stickiest product, lowest batch size, most difficult equipment)
- Validated sampling techniques — typically swab and rinse methods with defined recovery rates
- Clear acceptance criteria — based on MACO/PDE calculations, not “visually clean only”
| Aspect | Modern Regulatory Expectation (EMA, PIC/S, FDA) | Outdated / No Longer Accepted |
|---|---|---|
| Residue limits | PDE / ADE-based MACO (toxicology justified) | Arbitrary 10 ppm or visually clean only |
| Scope | All product-contact surfaces including worst-case areas | Only easy-to-access or visible areas |
| Sampling method | Validated swab & rinse with recovery study | Visual inspection only |
| Lifecycle | Periodic verification + revalidation triggers | One-time validation with static protocol |
| Worst-case selection | Based on solubility, toxicity, stickiness, batch size | Based only on product availability |
Modern regulators expect cleaning validation to be risk-based, scientifically justified, and lifecycle-managed, with periodic verification, revalidation triggers, change control impact assessment, and not treated as a one-time exercise.
Validation of Computerized Systems (CSV / CSA)
Validation of computerized systems ensures that GMP-relevant software and digital systems operate reliably, accurately, securely, and in full compliance with data integrity and regulatory requirements.
This applies to systems used in manufacturing, testing, quality decisions, batch release, traceability, and electronic data recording where any failure or uncontrolled behavior could negatively impact product quality, patient safety, or regulatory compliance.
Regulatory expectations are defined primarily by:
- EU GMP Annex 11 — lifecycle-based approach, control of suppliers, data integrity, security
- FDA 21 CFR Part 11 — electronic records and electronic signatures
- FDA CSA Guidance (Computer Software Assurance, 2025) — risk-based, assurance-driven alternative to traditional heavy CSV
- GAMP 5 (2nd Edition) — practical industry framework for categorization, documentation strategy, and risk-based testing
SEE ALSO: CSV vs CSA
Key validation focus points include:
- Functionality — does the system perform as intended for its GMP purpose?
- Data Integrity (ALCOA++) — accuracy, security, traceability, audit trails, access control, timestamp integrity
- Risk-Based Testing — focus on high-impact GMP functions, not generic IT functionalities
- Supplier Assessment — evaluation of vendor development practices, quality system, and documentation
- Electronic Records & Signature Compliance — controls over user roles, privileges, signature meaning, and tamper protection
- Change Control and Lifecycle Maintenance — validation is not “once and done”, ongoing impact evaluation is required
Modern regulators favor the CSA approach (FDA) and Annex 11 risk-based control model over the old “excessive documentation CSV” philosophy. The goal is assurance, not paperwork, meaning critical GMP functions receive the highest validation intensity, while low-risk and non-GMP functions are not over-validated.
Key Differences Between Qualification and Validation
Qualification and validation are often mentioned together, but in modern GMP enforcement they represent two distinct forms of regulatory evidence with different intent, timing, and lifecycle responsibilities.
| Aspect | Qualification | Validation |
|---|---|---|
| Primary Focus | Equipment, utilities, facilities, or computerized systems | Manufacturing process, analytical method, cleaning procedure, or computerized system performance over time |
| Purpose | Demonstrates that the system is technically ready and fit for intended use | Demonstrates that the process consistently delivers compliant product/results under routine operation |
| Timing | Completed before the system is released for GMP operation | Initiated only after qualification is complete and maintained throughout the lifecycle |
| Key Question Answered | “Is this system installed and functioning correctly according to its URS and design?” | “Can this process or method deliver reproducible results batch after batch within defined acceptance criteria?” |
| Example Activities | IQ, OQ, PQ of equipment or utility systems | Process Validation (PPQ/CPV), Cleaning Validation, Analytical Method Validation, CSV/CSA |
| Lifecycle Expectation | Mainly initial approval with periodic review or requalification | Ongoing lifecycle control — continued verification, trending, change control triggers |
| Regulatory Basis | EU GMP Annex 15 (qualification), Annex 11 (for computerized systems), WHO TRS 1019 | EU GMP Annex 15, FDA Process Validation Guidance, ICH Q2, Annex 11 / FDA CSA (for computerized systems) |
Regulatory Expectations for Qualification and Validation
Qualification and validation are not interpreted differently by chance, the world’s major regulatory authorities are fully aligned on the lifecycle-based, risk-driven expectation. However, each authority expresses this through its own guidance structure, which means understanding their intent, not just the wording, is critical for compliant execution.
The following sections highlight how the key global regulators position qualification and validation:
EU GMP Annex 15 & Annex 11
EU GMP Annex 15 defines the formal expectations for qualification and validation across the entire lifecycle, making it clear that qualification must be completed first before any process validation is initiated.
Annex 15 mandates a risk-based, scientifically justified approach, and explicitly states that validation is not a one-time event, but must be maintained through continued verification, periodic review, and change control reassessment.
Annex 11, which governs computerized systems, further establishes that data integrity, system security, and control of electronic records must already be ensured during qualification, before the system is used for any GMP-relevant process.
The Draft Revision of Annex 11 strengthens this further by:
- Emphasizing AI, cloud, and SaaS risk classification
- Requiring justification if suppliers are relied upon without independent testing
- Clarifying that GxP impact drives validation rigor, not system complexity
- Reinforcing the need for continuous oversight of system performance and data integrity, not initial approval alone
FDA Process Validation & Computer Software Assurance (CSA)
In the United States, the FDA Process Validation Guidance (2011) defines validation as a three-stage lifecycle, not a one-time batch exercise:
- Stage 1 – Process Design (development phase; process understanding and control strategy defined)
- Stage 2 – Process Performance Qualification (PPQ) (historic equivalent of “traditional validation”—but now must incorporate risk logic, statistical control, and reproducibility of commercial-scale execution)
- Stage 3 – Continued Process Verification (CPV) (ongoing monitoring using trend data, not a one-off approval)
FDA places heavy emphasis on scientific justification and objective evidence, especially statistical evidence of process capability over time.
For computerized systems, the FDA is phasing out traditional CSV in favor of Computer Software Assurance (CSA), which was officially finalized in September 2025. This modernizes validation expectations by requiring that:
- Testing prioritizes high-risk, GMP-critical functionality first
- Evidence must demonstrate process assurance, not just system compliance
- Vendor documentation may be leveraged, if risk-justified and properly assessed
ICH Q2 and ICH Q14
ICH Q2 defines the validation characteristics required to demonstrate that an analytical procedure is suitable for its intended purpose. The current revision (Q2(R2)), together with ICH Q14 on Analytical Procedure Development, embeds method validation within a lifecycle approach.
What inspectors expect you to show:
- The procedure’s role in the control strategy and why its accuracy, precision, specificity, range, LOD/LOQ, linearity, and robustness are appropriate for that role.
- Verification or transfer packages when a compendial method is adopted, proving suitability under local conditions.
- Ongoing performance evaluation: trending of system suitability, out-of-trend method behavior, and revalidation triggers after changes to method, equipment, or matrices.
GAMP 5
GAMP 5 (2nd ed.) provides the industry framework for computerized systems. It emphasizes:
- Lifecycle and supplier involvement: define URS, assess supplier quality practices, and maintain traceability through the V-model.
- Risk-based testing: concentrate evidence on functions with GMP impact; avoid testing commodity or low-risk features beyond what assurance requires.
- Documentation proportionality: right-sized deliverables that support assurance, consistency, and auditability without redundant paperwork.
- Operational control: change control, periodic review, incident/deviation handling, and data integrity monitoring to sustain the validated state.
GAMP 5 aligns naturally with FDA CSA and Annex 11: assurance first, documentation that serves assurance, and testing intensity driven by GxP impact.
See Also: Purpose of GAMP 5 in CSV
FAQ
Why Is Qualification Considered Part of Validation in Some Regulatory Frameworks?
EU GMP Annex 15 clearly states that qualification is a subset of validation because it forms the technical foundation on which process validation is built. FDA follows the same interpretation through its lifecycle model, qualification evidence feeds directly into Stage 2 PPQ.
However, qualification and validation are still treated differently in terms of timing and purpose. Qualification confirms equipment readiness; validation confirms process performance. They are connected, but not interchangeable.
Can Qualification and Validation Be Performed Simultaneously?
In some cases — Yes, but only with strong documented justification. For example, IQPQ or combined FAT/SAT + PQ may be performed when time is critical, such as during tech transfer or essential market supply.
However, simultaneous execution is still expected to maintain clear logical separation between system capability (qualification) and process consistency (validation). Regulators will immediately flag any blending that suggests shortcutting rather than efficiency. It is acceptable only when risk-based and justified in the Validation Master Plan (VMP).
What Happens if Validation Is Done Before Qualification Is Complete?
Any validation performed on unqualified equipment or utilities is considered invalid by regulators, regardless of how well it is documented. This is a common cause of FDA 483s and EU inspection observations.
The logic is simple: you cannot prove consistent process performance when the equipment’s capability has not even been confirmed. In such cases, revalidation under proper lifecycle control is unavoidable. This often results in major delays and remediation costs.
Can Vendor Documentation Replace Qualification and Validation Activities?
Vendor documentation can be leveraged but not blindly accepted. Regulators expect a supplier assessment (Annex 11, CSA, GAMP 5) that proves the vendor follows a robust quality system.
However, critical GMP functionality must still be independently challenged and verified by the manufacturer. This is especially true for alarm behavior, security, audit trails, and fail-safe logic. Leveraging vendor evidence is intelligence, relying without assessment is negligence.
What Is the Role of the Validation Master Plan (VMP) in Qualification vs Validation?
The VMP defines the strategic framework for both qualification and validation lifecycle control. It explains what requires qualification, what requires validation, in what order, and why.
It also defines the risk prioritization rules, revalidation triggers, and documentation expectations. In regulatory inspections, the VMP is often reviewed first, because it reveals whether the company’s approach is proactive, traceable and risk-based, or reactive and ad-hoc.
Do Computerized Systems Require Both Qualification and Validation?
Yes, computerized systems require qualification of the infrastructure and platform, plus validation/CSA of the GMP-critical functions and workflows. EU Annex 11 and FDA 21 CFR Part 11 require both aspects, technical readiness + process assurance.
Qualification alone is never sufficient. Regulators expect functional testing based on data integrity and decision impact, not just infrastructure setup evidence.
Can a Process Be Moved to Commercial Use if Validation Is Only Partially Complete?
Only under strictly justified, risk-assessed, and regulator-approved exceptional conditions, typically for medically critical product shortages or public health emergencies. In such cases, concurrent validation may be used, but every decision must be documented, pre-approved in the VMP, and monitored batch-by-batch. This is not a shortcut; it is an emergency exception requiring extremely strong control.
How Often Should Qualification and Validation Be Repeated?
Requalification and revalidation are not based on fixed calendar intervals, but on risk-based triggers such as equipment modification, process deviation, trend shifts, change in suppliers, or regulatory updates.
Periodic review is still expected to confirm continued suitability, but re-execution only when justified. The goal is evidence-based lifecycle assurance, not wasteful rework.
What if Qualification Identifies Minor Deviations - Can Validation Still Proceed?
Only if deviations are documented, risk-assessed, and formally accepted by QA prior to progression. If the impact is non-critical, justified, and controlled, validation can proceed with documented rationale. If the issue is GMP-critical (e.g., missing calibration, reject sensor failure, data integrity control not functional), validation must be stopped until corrected.
Final Takeaway
Qualification and validation are not simply procedural requirements, they are the regulatory foundation of evidence-based manufacturing control. Qualification ensures that the equipment, utilities, and systems are technically capable and GMP-ready, while validation proves that the process, method, or computerized function will continue to perform consistently and safely over time.
Global regulators are fully aligned: lifecycle control, risk-based justification, and traceable scientific reasoning are non-negotiable. Validation is no longer accepted as a one-time event. Every decision must be defensible, data-backed, and continuously verifiable.
In short:
Qualification makes a system fit for GMP use. Validation keeps it trusted for patient use — every day, not just on Day 1.
Companies that manage qualification and validation as continuously maintained control systems, not one-time documentation exercises, are the ones that pass inspections, scale globally, and stay confident under regulatory pressure.















