Quality by Design (QbD) is a structured approach to pharmaceutical development that focuses on understanding processes and controlling variability from the start. It helps teams design products that consistently meet quality standards by linking critical material attributes and process parameters to final product performance.
Guided by ICH Q8(R2), Q9, and Q10, QbD shifts attention from reactive quality control to proactive design and development. It fosters a deeper understanding of how ingredients, equipment, and processing conditions impact safety and efficacy, particularly during scale-up and routine manufacturing.
This article brings together core concepts and practical tools from leading QbD resources to support pharmaceutical teams working in R&D and GMP. The goal is straightforward: fewer problems, better products, and a simpler path to regulatory approval.
What Is Quality by Design?

Quality by Design (QbD) is a systematic framework for pharmaceutical development that focuses on building quality into a product from the earliest stages. Instead of relying solely on final product testing, QbD emphasizes process understanding, risk assessment, and control strategies to ensure that products consistently meet their intended performance.
The concept was formalized through ICH guidelines, most notably:
- ICH Q8(R2): Pharmaceutical Development
- ICH Q9: Quality Risk Management
- ICH Q10: Pharmaceutical Quality System
At its core, QbD asks three key questions:
- What are we trying to achieve? (Quality Target Product Profile – QTPP)
- What factors can impact quality? (Critical Quality Attributes – CQAs)
- How can we control the process to ensure consistent quality? (Design Space, Control Strategy)
QbD doesn’t just apply to new drug development; it supports continuous improvement across the entire product lifecycle. By identifying risks early and using data to guide decisions, QbD reduces failures, shortens development timelines, and strengthens regulatory submissions.
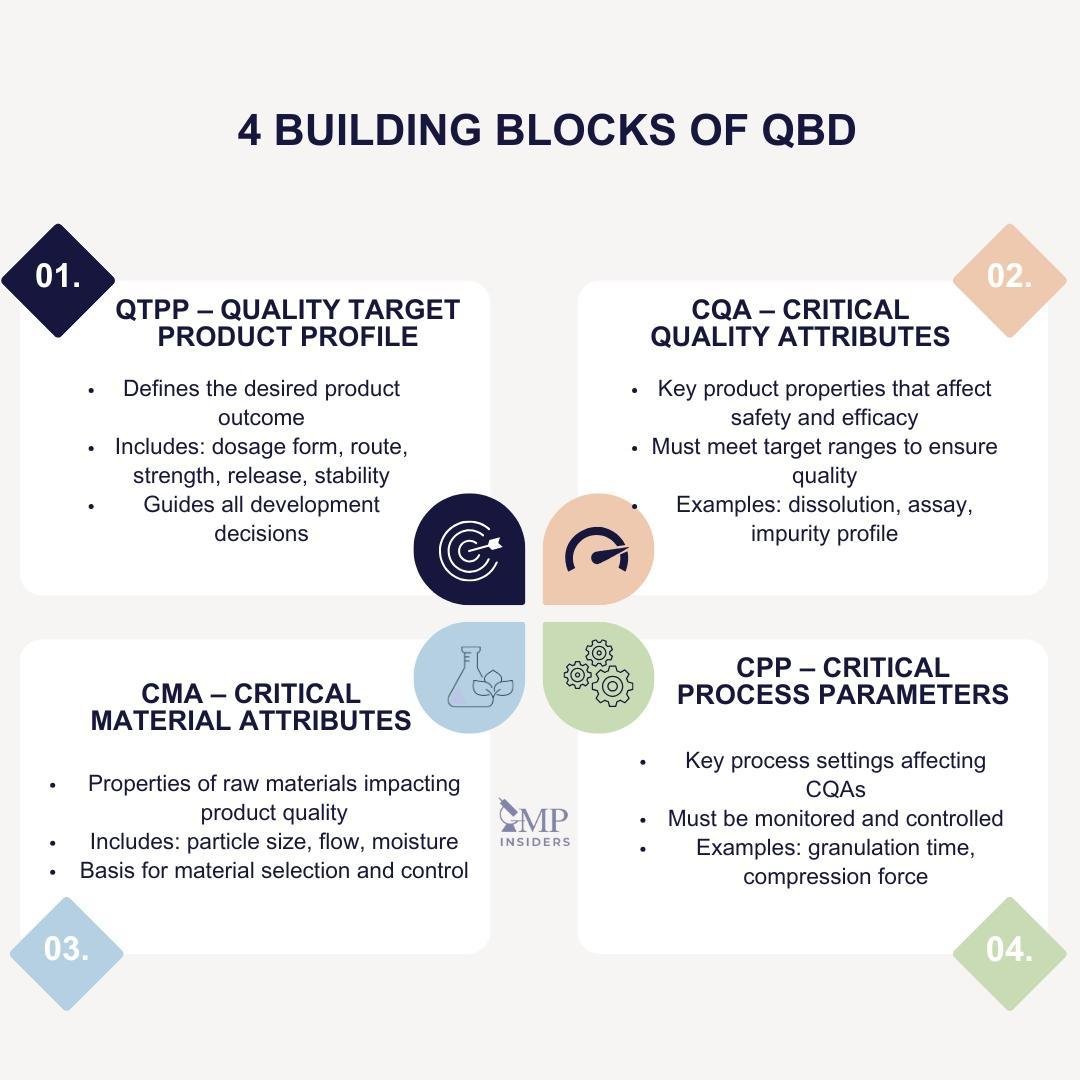
Main Building Blocks of QbD
Quality by Design relies on a structured set of elements that support the development of robust and consistent pharmaceutical products. These building blocks help define, understand, and control the formulation and process, ensuring the final product performs as intended.
Below, we illustrate each concept using a single dosage form: an oral modified-release tablet.
1. Quality Target Product Profile (QTPP)
The QTPP is the starting point of any QbD strategy. It outlines the intended quality characteristics of the final product, including its dosage form, route of administration, strength, therapeutic effect, and performance attributes such as dissolution rate or stability. Essentially, the QTPP defines what success looks like for a finished product from the patient’s perspective.
By clearly defining the QTPP early in development, pharmaceutical teams can align formulation strategies, analytical methods, and manufacturing processes with the desired outcomes.
Example QTPP for oral modified-release tablet:
- Dosage form: modified-release tablet
- Route: oral
- Strength: 500 mg
- Release profile: 80% release over 12 hours
- Stability: minimum 24-month shelf life under ICH Zone II conditions
2. Critical Quality Attributes (CQAs)
Once the QTPP is established, the next step is to identify the Critical Quality Attributes (CQAs), which are the physical, chemical, biological, or microbiological properties that must be controlled to ensure the product meets its intended quality. CQAs are typically linked to safety, efficacy, and performance.
Identifying CQAs requires a scientific understanding of the product and its intended use, often supported by prior knowledge, literature, and experimental data.
Example CQAs for oral modified-release tablet:
- Dissolution rate (e.g., not less than 80% in 12 hours)
- Assay (content of active substance)
- Impurity profile (below specified thresholds)
- Tablet hardness (to ensure mechanical integrity)
- Moisture content (to prevent degradation)
3. Critical Material Attributes (CMAs)
CMAs are the key characteristics of raw materials, including the active pharmaceutical ingredient (API) and excipients, that can influence the final product quality. Understanding the CMAs helps ensure that any variability in raw materials does not adversely impact the CQAs.
Control of CMAs typically begins with supplier qualification and continues through the specification of raw materials, testing, and handling procedures.
Example CMAs for oral modified-release tablet:
- API particle size (influences dissolution rate)
- Polymer grade and viscosity (affects matrix formation)
- Moisture content of excipients (impacts tablet stability)
- Flow properties of the blend (affects uniformity of fill and compression)
4. Critical Process Parameters (CPPs)
CPPs are the process variables that directly affect CQAs. These parameters must be identified, understood, and controlled to maintain process consistency and product quality. A slight deviation in a CPP could lead to an out-of-specification (OOS) result, batch failure, or increased risk to patient safety.
The relationship between CPPs and CQAs is typically studied using design of experiments (DoE), process mapping, and risk assessment tools such as FMEA.
Example CPPs for oral modified-release tablet:
- Granulation end-point (determines blend uniformity and tablet strength)
- Compression force (affects hardness and dissolution)
- Coating spray rate and temperature (critical for release-modifying coatings)
- Drying time (influences residual moisture and stability)
How These Elements Work Together in QbD
In QbD, these elements are interdependent. For example, a change in the polymer viscosity (CMA) might require an adjustment in the coating process (CPP) to maintain the desired dissolution profile (CQA) aligned with the QTPP. Understanding these relationships enables the development of a scientifically sound design space and an effective control strategy.
| QTPP elem. | CQA | CMA/CPP drivers | Studies / models | Proven acceptable ranges / Design space | Controls (IPCs/PAT) | Release spec | CPV metric |
|---|---|---|---|---|---|---|---|
| 80% release @12 h | Dissolution profile | Polymer grade/viscosity (CMA); compression force, coating temp (CPP) | 2³ factorial + RSM; regression | DS: polymer 12–18%, force 5–8 kN, granulation 10–15 min | NIR for blend, in‑process hardness, coat weight | Q = 80% ± window | Cpk≥1.33; EWMA chart on f₂ surrogate |
This integrated approach allows development teams to anticipate variability, reduce risk, and deliver consistent product quality from early development through commercial manufacturing.
Risk Management Embedded in Quality by Design
Risk management is a central element of Quality by Design. It enables pharmaceutical teams to identify which material attributes and process parameters have the most significant impact on product quality, and to focus resources on controlling those variables.
Rather than treating all risks equally, QbD applies a structured approach to evaluate, prioritize, and mitigate potential issues before they reach commercial production.
SEE ALSO: Quality Risk Management in Pharmaceutical Industry
Key Risk Management Tools in QbD
Several formal tools are commonly used to support risk-based decision-making:
- Failure Mode and Effects Analysis (FMEA): Identifies potential failure points in a process or product and ranks them based on severity, occurrence, and detectability.
- Ishikawa (Fishbone) Diagrams: Helps visualize the root causes of a potential quality issue by mapping out contributing factors such as materials, equipment, personnel, and methods.
- HACCP (Hazard Analysis and Critical Control Points): Focuses on identifying critical control points to prevent product defects or safety risks.
These tools are used iteratively throughout the development process, from early formulation studies to scale-up and commercial production.
Applying Risk Management: A Practical Example
In the case of a modified-release tablet, suppose the development team is concerned about the variability in drug release. A risk assessment might reveal:
- High-risk CMA: Polymer viscosity variability affecting matrix integrity
- High-risk CPP: Compression force influencing tablet porosity and release rate
- Medium-risk factor: API particle size distribution
With this information, the team can design experiments (e.g., DoE) to study the impact of these variables on dissolution and optimize the formulation accordingly. Later, the same data supports the justification of a design space, where the process can operate with flexibility while maintaining product quality.
| Activity | Risk to patient/supply | Data maturity | Formality level | Approvals / records |
|---|---|---|---|---|
| Early CQA screen | Medium | Low | Medium (FMEA + SME review) | Dev QA sign‑off |
| Design space justification | High | Medium–High | High (DoE, models, verification batches, statistical review) | QA/QP; regulatory filing |
| Minor excipient supplier change within DS | Low | High | Low–Medium (risk check + CPV watch) | Change control |
Design Space and Control Strategy in QbD
After identifying the key material and process variables that influence product quality, the next step in a Quality by Design (QbD) framework is to define the boundaries within which the process can reliably operate; this is known as the design space.
To ensure that operations remain within these boundaries during manufacturing, a robust control strategy must also be developed. Together, these elements translate process understanding into consistent, high-quality outcomes.
What Is Design Space?
Design space, as defined in ICH Q8(R2), is the multidimensional combination of input variables, such as critical material attributes (CMAs) and critical process parameters (CPPs), that have been proven, through scientific studies, to ensure product quality.
Operating within this space offers flexibility in manufacturing without requiring regulatory re-approval for minor process adjustments.
Example: For a modified-release oral tablet, a validated design space may include:
- Polymer concentration: 12–18%
- Compression force: 5–8 kN
- Granulation time: 10–15 minutes
These conditions ensure that the drug release profile and stability remain within defined specifications.
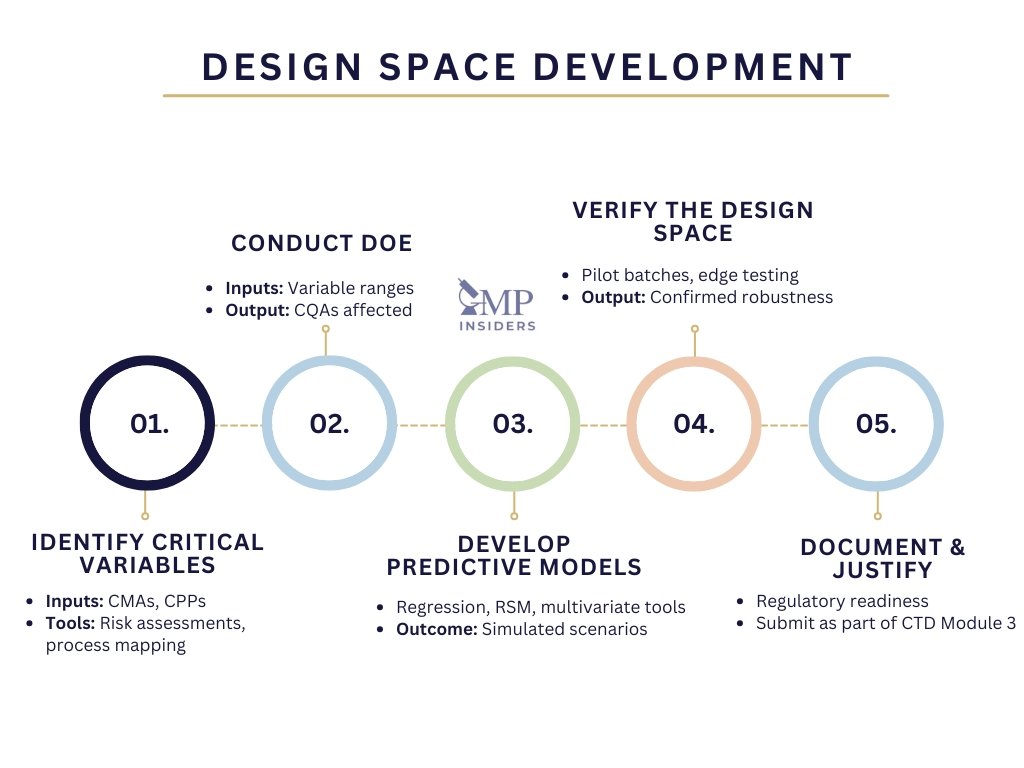
1. Identify Critical Variables
The first step is to identify which Critical Material Attributes (CMAs) and Critical Process Parameters (CPPs) have the potential to impact Critical Quality Attributes (CQAs) of the product.
This is done through:
- Prior knowledge from literature, legacy products, or platform technologies
- Process mapping to understand unit operations
- Risk assessment tools to assess the variability across categories like materials, equipment, personnel, and environment
2. Conduct Design of Experiments (DoE)
Once key variables are identified, the next step is to conduct a Design of Experiments (DoE) to understand how these factors and their interactions influence product quality.
This involves:
- Choosing an appropriate experimental design (e.g., full factorial, central composite, or Box-Behnken)
- Systematically varying input parameters across a range of values
- Collecting data on CQAs (e.g., dissolution, hardness, friability)
The DoE provides the statistical foundation for quantifying how sensitive the process is to each variable, both independently and in combination with other variables.
Tip: A well-structured DoE saves time and resources while revealing non-obvious relationships between parameters.
3. Develop Predictive Models
The data from the DoE is used to create statistical or mathematical models that predict how different combinations of variables will affect the product’s CQAs. These models are used to:
- Define acceptable operating ranges
- Identify design space boundaries
- Assess the robustness and sensitivity of the process
Regression models, response surface methodology (RSM), and multivariate analysis tools are often employed to develop these models.
Outcome: You can simulate process performance under various conditions before physically running the batches.
4. Verify the Design Space
Model predictions must be verified through experimental confirmation, typically by manufacturing pilot-scale or validation batches under the proposed design space conditions. This step ensures that the model accurately reflects real-world behavior.
Activities may include:
- Producing batches at edge-of-range parameter settings
- Verifying that CQAs remain within acceptable limits
- Documenting batch performance and variability
This step is crucial for building confidence before full-scale commercial implementation.
5. Document and Justify
Once verified, the design space must be thoroughly documented and justified for inclusion in regulatory submissions (e.g., CTD Module 3).
This includes:
- Descriptions of the risk assessments conducted
- Experimental design details and statistical analysis
- Model summaries and process performance data
- Defined ranges for critical parameters
- A scientific rationale showing how the design space ensures product quality
When properly justified, changes made within the design space typically do not require regulatory approval, thereby allowing for greater flexibility in manufacturing.
What Is a Control Strategy?
A control strategy is a planned set of controls that ensures product quality is maintained during routine manufacturing. It’s developed based on process understanding and is designed to monitor and manage variability within the design space.
It includes:
- Raw Material Controls: Specifications for API and excipients (e.g., moisture content, particle size)
- In-Process Controls (IPCs): Parameters monitored during manufacturing (e.g., tablet weight, hardness, granule moisture)
- Process Analytical Technology (PAT): Real-time monitoring tools (e.g., NIR spectroscopy for blend uniformity)
- Finished Product Testing: Final verification of CQAs (e.g., assay, dissolution, impurities)
- Equipment and Environmental Controls: Conditions like drying temperature, humidity, and machine settings
| Control Element | Examples | Monitored Parameter | Stage |
|---|---|---|---|
| Raw Material Control | Excipient spec, moisture control | Moisture, particle size | Before manufacturing |
| In-Process Control (IPC) | Blend uniformity, tablet weight | Tablet hardness, weight | During compression |
| Process Analytical Tech | NIR for blend uniformity | Real-time spectral data | In-line/at-line |
| Finished Product Testing | Dissolution, assay | API content, impurities | Final release |
| Equipment/Env Monitoring | Temperature/humidity logging | HVAC, coating temp | All stages |
Benefits of Defining Both Elements
When implemented together, design space and control strategy offer significant technical, regulatory, and operational advantages:
- Operational Flexibility: Adjustments within the design space can be made without submitting a variation, saving time and cost.
- Improved Process Robustness: Processes are more stable and resilient, reducing the likelihood of deviations or batch failures.
- Faster Tech Transfers: Having a defined space and controls simplifies site transfers and scale-up during commercialization.
- Regulatory Confidence: Demonstrates that the process is built on science and risk management, enhancing the quality of regulatory submissions.
- Supports Continuous Improvement: Real-world process data can be used to refine the design space and controls over time, aligning with ICH Q12 principles for lifecycle management.
Lifecycle Management and Continued Process Verification (CPV)
Establishing a robust manufacturing process through Quality by Design (QbD) doesn’t end with regulatory approval. Maintaining product quality over time requires active lifecycle management, supported by tools like Continued Process Verification (CPV). This phase ensures that the process remains in a state of control as it moves into routine commercial production.
Why Lifecycle Management Matters
Even well-characterized processes can drift over time due to changes in raw materials, equipment wear, environmental conditions, or human factors. Lifecycle management offers a structured approach to monitoring, evaluating, and continually improving processes, thereby reducing the risk of deviations and ensuring sustained product quality.
In the context of ICH Q10 and Q12, lifecycle management supports:
- Ongoing verification of process performance
- Data-driven decision-making for process improvements
- Smoother implementation of post-approval changes
What Is Continued Process Verification (CPV)?
CPV is the third stage of process validation (following Stage 1: Process Design and Stage 2: Process Qualification). Its purpose is to confirm that the manufacturing process continues to perform as intended during routine production.
CPV involves:
- Real-time and batch-by-batch data monitoring: Collecting and trending key process and quality data (e.g., yield, hardness, assay, dissolution)
- Use of statistical tools: Control charts, trend analysis, and capability indices are applied to detect early signs of variability
- Feedback loops for continuous improvement: If trends suggest a potential drift, corrective actions or process refinements can be introduced proactively
SEE ALSO: Process Validation Lifecycle: A Risk-Based Approach
Integration with QbD Principles
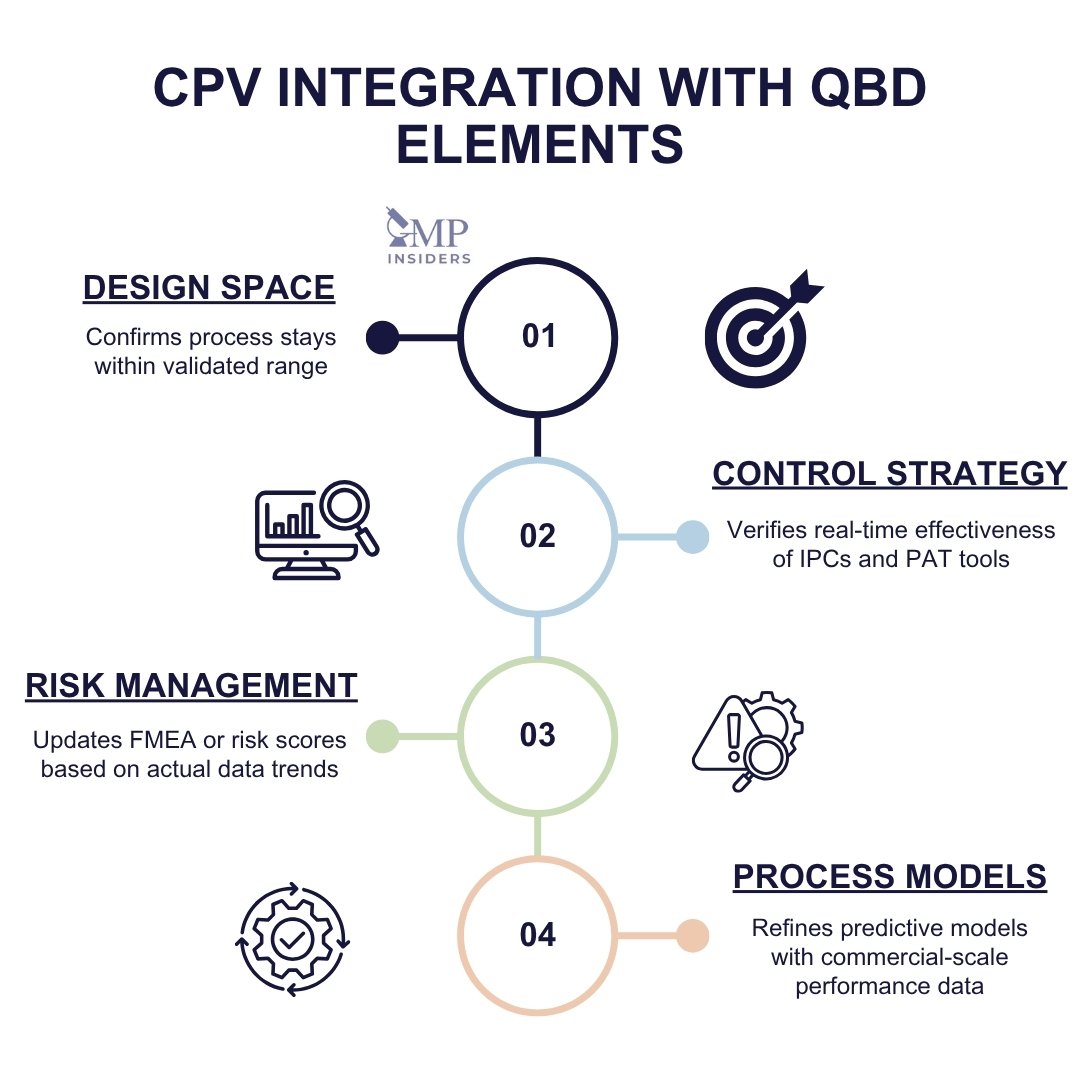
CPV reinforces the foundation built during QbD development by:
- Validating that operations stay within the design space
- Confirming the effectiveness of the control strategy
- Supporting updates to risk assessments and control limits based on real-world performance
As more data is collected, the process model becomes stronger, and opportunities for optimization, such as tightening specifications or expanding the design space, can be explored.
Regulatory Requirements for Quality by Design
Quality by Design (QbD) is not a regulatory obligation, but once adopted and referenced in a submission, it becomes a formal commitment that must meet clearly defined regulatory expectations.
Authorities, including the FDA, EMA, and other ICH member countries, encourage the use of QbD because it enhances product understanding, supports risk-based development, and improves lifecycle control.
Several ICH guidelines collectively define the framework for QbD, including expectations for risk assessment, process design, control strategies, and post-approval change management.
ICH Q8(R2): Pharmaceutical Development
The concept of design space is formally introduced and defined in ICH Q8(R2). Key regulatory positions include:
Definition:
Quality by Design (QbD): A systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management.
Key regulatory expectations under ICH Q8(R2):
- Design Space Definition: Companies may choose to define a design space, a multidimensional range of material attributes and process parameters that have been proven to ensure product quality. Once approved, this space becomes binding.
- Regulatory Flexibility: Changes made within an approved design space do not require post-approval regulatory notification, offering more operational freedom.
- Submission Requirements: If a design space is proposed in the Common Technical Document (CTD, Module 3), it must be supported by scientific data, process models, and risk assessments.
- Optional but Binding: Including QbD and design space elements is voluntary, but once approved, regulatory agencies expect strict adherence unless formally revised through change control procedures.
ICH Q9: Quality Risk Management
ICH Q9 outlines how risk-based thinking should be embedded in pharmaceutical development, forming the core of Quality by Design (QbD) decision-making.
Regulatory expectations under ICH Q9:
- Identification and Prioritization of Risk: Companies must demonstrate a structured approach to assessing how input variables (CMAs and CPPs) impact critical quality attributes (CQAs).
- Use of Risk Tools: Common tools include Failure Mode and Effects Analysis (FMEA), Ishikawa diagrams, risk matrices, and Pareto charts.
- Traceability: All risk assessments must be documented and traceable to development decisions, process controls, and justification of ranges in the design space.
Without a strong risk management foundation, regulators are unlikely to accept a proposed design space or control strategy.
ICH Q10: Pharmaceutical Quality System
ICH Q10 provides the framework for managing QbD outcomes across the product lifecycle, ensuring that what is established during development is effectively maintained in commercial production.
Regulatory expectations under ICH Q10:
- Integration with the Pharmaceutical Quality System (PQS): QbD outputs, such as design space and control strategies, must be embedded into the quality system and reflected in SOPs, training, and change control.
- Ongoing Process Verification (OPV/CPV): Companies must implement continued process verification (CPV) to monitor whether the process remains in control over time.
- Change Control and Knowledge Management: Lifecycle changes based on QbD findings should be handled under formal change control procedures, supported by real-time data and clear justification.
Post-Approval Changes (ICH Q12)
ICH Q12 builds on the principles of QbD and outlines how companies can manage post-approval changes efficiently using science- and risk-based protocols.
Regulatory expectations under ICH Q12:
- Post-Approval Change Management Protocols (PACMPs): QbD elements, such as expanding the design space or updating control strategies, can be pre-approved through PACMPs, allowing for faster implementation without requiring complete regulatory resubmission.
- Structured Knowledge Transfer: Companies must document how Quality by Design (QbD) knowledge gained during development supports change management throughout the product lifecycle.
- Use of Real-World Data: Continued monitoring of process performance supports updates to QbD elements and justifies change requests.
ICH Q12 aims to reduce regulatory burden without compromising quality, making QbD an enabler of faster, controlled innovation.
FAQ
Can QbD Be Applied to Generic Drug Development?
Yes, QbD is increasingly applied in the development of generics. It helps generic manufacturers demonstrate bioequivalence and process robustness more efficiently. Understanding critical parameters early can also speed up development timelines and reduce costs.
Regulatory bodies, such as the FDA, have issued specific guidance for implementing Quality by Design (QbD) in generic submissions. The use of QbD can also support risk-based approaches in Abbreviated New Drug Applications (ANDA) filings.
What Are the Key Documents Produced During QbD Implementation?
Common QbD documentation includes the Quality Target Product Profile (QTPP), risk assessments (e.g., FMEA), Design of Experiments (DoE) protocols and results, statistical models, design space justifications, and control strategy descriptions. These documents support the regulatory submission and internal process validation.
They form the technical foundation for ongoing lifecycle management. Regulatory agencies expect traceability between development data and the final product specifications. Proper documentation ensures continuity across teams and stages.
What Is the Role of PAT in QbD?
Process Analytical Technology (PAT) supports real-time measurement and control of critical process parameters. In a QbD framework, PAT enables continuous monitoring to ensure processes remain within the design space.
It reduces reliance on end-product testing and supports adaptive control strategies. PAT tools include spectroscopy (e.g., NIR), particle size analyzers, and real-time sensors. The use of PAT improves consistency, reduces cycle times, and facilitates continuous improvement.
Can QbD Be Applied to Biologics and Biosimilars?
Yes, QbD is highly applicable to biologics, including biosimilars. These complex products benefit from in-depth process understanding due to their sensitivity to variability. QbD helps define key attributes such as glycosylation patterns, protein folding, and aggregation.
It also supports control strategies that minimize batch-to-batch differences. Regulatory agencies often require a Quality by Design (QbD)- based approach for large molecule submissions.
What Is the Difference Between Design Space and Proven Acceptable Ranges?
A design space is a multidimensional range of variables that has been proven to ensure product quality through scientific data and modeling. Proven acceptable ranges (PARs), on the other hand, are narrower and established based on limited data or experience.
Operating within a designated design space allows for flexibility without requiring regulatory notification. Changes within PARs still often require justification and may trigger variations. Design space is broader and formally recognized in regulatory filings.
Can a Design Space Be Modified Post-Approval?
Yes, but any modification to the design space requires regulatory evaluation unless the change falls within the scope of an approved Post-Approval Change Management Protocol (PACMP).
ICH Q12 facilitates such lifecycle changes when pre-defined protocols are in place. Companies must submit updated data to justify new ranges. Without PACMP, any shift outside the original space may be considered a variation or a major change. Planning is key to enabling flexible post-approval updates.
Can QbD Be Applied Retrospectively to Existing Products?
Yes, QbD principles can be retroactively applied to legacy products, especially during lifecycle management or when facing process variability issues. Retrospective QbD efforts focus on building process understanding and redefining control strategies.
This may be required to support post-approval changes or modernization of manufacturing practices. However, applying QbD retrospectively can be a resource-intensive process. It is most effective when paired with robust data from historical production.
How Does QbD Influence Analytical Method Development?
QbD principles are applied to analytical method development under the concept of Analytical Quality by Design (AQbD). This includes defining an Analytical Target Profile (ATP), identifying method variables, and utilizing Design of Experiments (DoE) to optimize method performance.
AQbD enhances method robustness and minimizes variability. It also supports lifecycle management of analytical procedures, especially under the new ICH Q14 guideline. AQbD ensures that analytical methods are fit for purpose throughout the product lifecycle.
Final Thoughts
Quality by Design is more than a regulatory framework; it’s a mindset that reshapes how pharmaceutical products are developed, scaled, and manufactured. By emphasizing process understanding, risk management, and control from the earliest stages, QbD enables companies to move beyond compliance and toward operational excellence.
Defining a clear design space and building a data-driven control strategy leads to more robust products, fewer failures, and smoother regulatory interactions. It also lays the foundation for continuous improvement, lifecycle flexibility, and innovation in both small-molecule and biologic development.
As the industry continues to adopt digital tools, real-time monitoring, and ICH Q12 lifecycle approaches, QbD remains a vital strategy for maintaining product quality and accelerating time to market. For R&D teams, quality units, and manufacturers alike, investing in QbD today means building a more resilient and efficient future for tomorrow’s medicines.














