The use of toxic solvents in Quality Control (QC) laboratories is a double-edged sword: while they are essential for ensuring the reliability and accuracy of drug testing, they also pose significant health and environmental risks. As QC analysts in the pharmaceutical industry are continuously exposed to these hazardous chemicals, it becomes crucial to explore strategies that maintain testing quality while minimizing the impact on human health and the environment.
As the industry advances, QC labs must balance their commitment to precise and consistent results with the responsibility of adopting greener practices. To ensure the quality of RP-HPLC results while protecting analysts and the environment, it is essential to prioritize the development of eco-friendly analytical methods and to reduce the use of toxic solvents in mobile phases through different approaches.
What Does RP-HPLC Mean?
Reverse Phase High-Performance Liquid Chromatography (RP-HPLC) is widely used for separating molecules based on their hydrophobicity, employing a nonpolar stationary phase, typically silica modified with alkyl chains, and a polar mobile phase. The separation is achieved using either gradient or isocratic elution methods.
RP-HPLC’s versatility and efficiency make it a preferred choice in industries like pharmaceuticals, but its widespread use is closely linked to the heavy consumption of toxic solvents, such as acetonitrile and methanol. While RP-HPLC is known for its versatility and reliability in various industries, including pharmaceuticals, the use of toxic solvents like acetonitrile and methanol requires careful handling and disposal to minimize environmental and health risks.
Which Toxic Solvents are Used in RP-HPLC?
In RP-HPLC, acetonitrile and methanol are the most commonly used solvents due to their effectiveness in separating organic compounds. However, both solvents are classified as toxic and pose significant environmental and health hazards.
Their widespread use in large volumes during the chromatography process, from equilibration to elution and column maintenance, underscores the need for proper management and disposal to minimize their impact on laboratory safety and sustainability efforts.
Using Acetonitrile and Methanol in RP-HPLC
Acetonitrile (ACN) and methanol stand out as widely used options, each offering unique properties that serve various applications in laboratories and industrial settings. While both solvents play crucial roles in processes such as extraction, chromatography, and synthesis, they also come with distinct advantages and disadvantages.
Acetonitrile
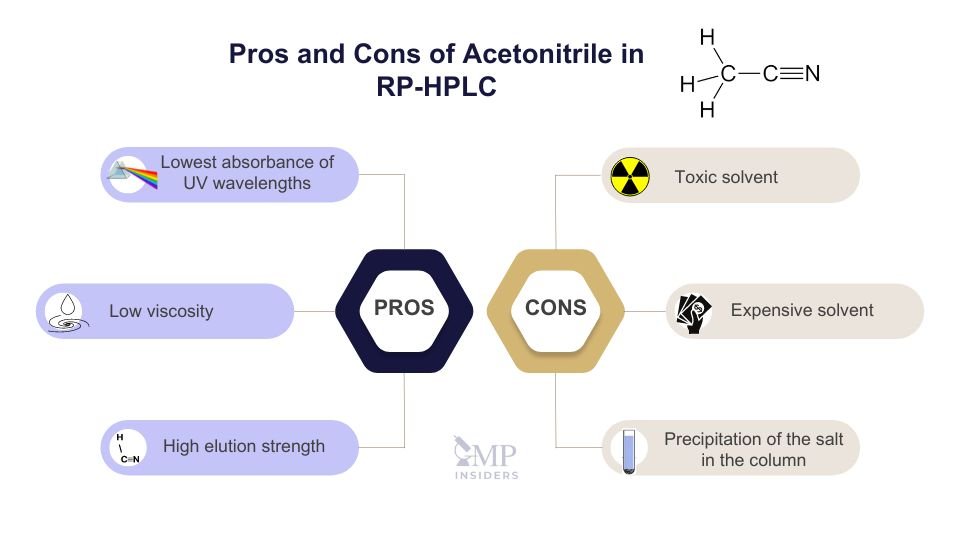
Acetonitrile is one of the most commonly used solvents in RP-HPLC due to its favorable properties, such as low UV absorbance and efficient flow through the column. Despite its advantages, acetonitrile presents several challenges, including toxicity and environmental concerns, which must be carefully managed.
Pros:
- Lowest absorbance of UV wavelengths, making it suitable for UV detection in HPLC.
- Efficient flow through the column, as a result of low viscosity.
- High elution strength, allowing for effective separation of analytes.
- Easily soluble in water, empowering the creation of diverse mobile phases.
- Its polarity aids in dissolving various ranges of analytes.
Cons:
- Expensive solvent.
- Considered more toxic and hazardous than some alternative solvents.
- Requires careful handling and disposal due to its toxicity.
- May have environmental implications if not properly managed.
Is Acetonitrile Genuinely That Harmful?
Acetonitrile is a commonly used solvent in RP-HPLC due to its beneficial properties, but its toxicity should not be underestimated. Laboratory analysts are often exposed to acetonitrile and methanol vapors, which can be harmful if inhaled. The level of toxicity depends on the route of exposure (skin contact, inhalation, etc.), as well as the amount and frequency.
Acute exposure to acetonitrile can cause irritation of mucous membranes when inhaled at concentrations around 500 ppm, as indicated by occupational safety guidelines. Higher exposure levels may lead to severe symptoms, such as convulsions, nausea, abdominal pain, and weakness.
Once absorbed, acetonitrile metabolizes into cyanide, which is toxic to the human body. This metabolic process is facilitated by the enzyme cytochrome P450, which converts acetonitrile into cyanide, emphasizing the risks associated with prolonged exposure. Chronic exposure could lead to cyanide poisoning, with symptoms such as headaches, paralysis, and tremors.
As a Group D chemical, acetonitrile is not currently classified as a human carcinogen by the EPA, although this classification may be re-evaluated if new evidence arises. Despite its lack of classification as a carcinogen, acetonitrile remains a hazardous substance that requires careful handling.
Methanol
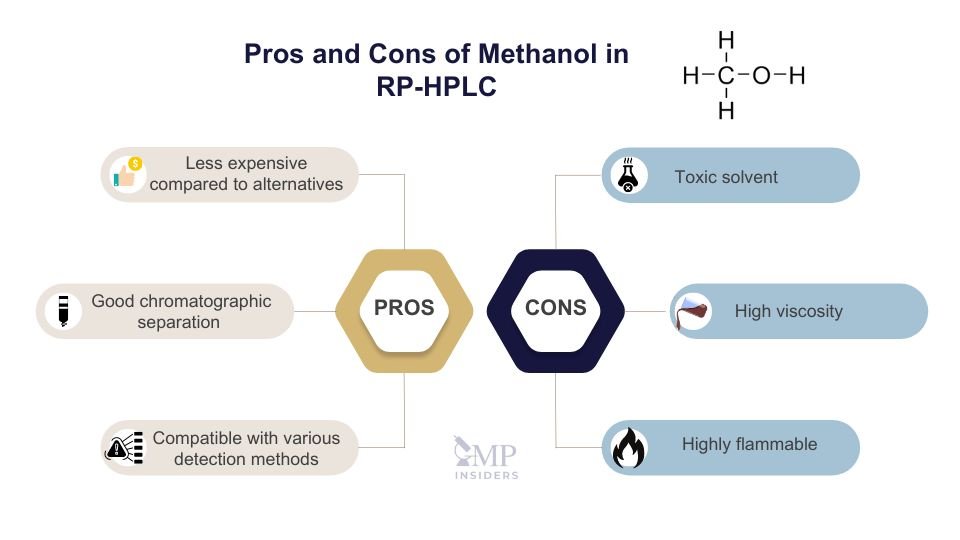
Methanol is another widely used solvent in RP-HPLC due to its cost-effectiveness and compatibility with various detection methods. Despite its advantages, methanol is toxic and flammable, necessitating strict safety measures during handling and disposal.
Pros:
- Effective for a wide range of analytical applications.
- Generally less expensive compared to acetonitrile.
- Provides good chromatographic performance and peak shape.
- Compatible with various detection methods, including UV and MS.
Cons:
- Higher viscosity compared to acetonitrile, potentially affecting flow rates.
- Toxic and flammable, requiring strict safety measures during handling and storage.
- Environmental concerns regarding its disposal and impact.
Toxicity of Methanol
Laboratory analysts are commonly exposed to methanol by inhalation, dermal exposure, and ingestion. Methanol is a highly flammable liquid and vapor and is also classified as toxic if in contact with skin, swallowed, or inhaled. Exposure to methanol can lead to irreversible effects, such as skin irritation, eye irritation, and respiratory tract irritation.
Most commonly, side effects include shortness of breath, headache, and nausea. Methanol can also lead to blindness or be fatal if swallowed.
According to the EPA, “No information is available on the carcinogenic effects of methanol in humans or animals.“ This absence of data indicates that current research is insufficient to classify methanol as either a carcinogen or non-carcinogen. However, its acute toxicity and potential for causing severe health effects make it a highly hazardous substance that requires strict safety measures.
A Comparison of Solvent Toxicity Between RP-HPLC and NP-HPLC
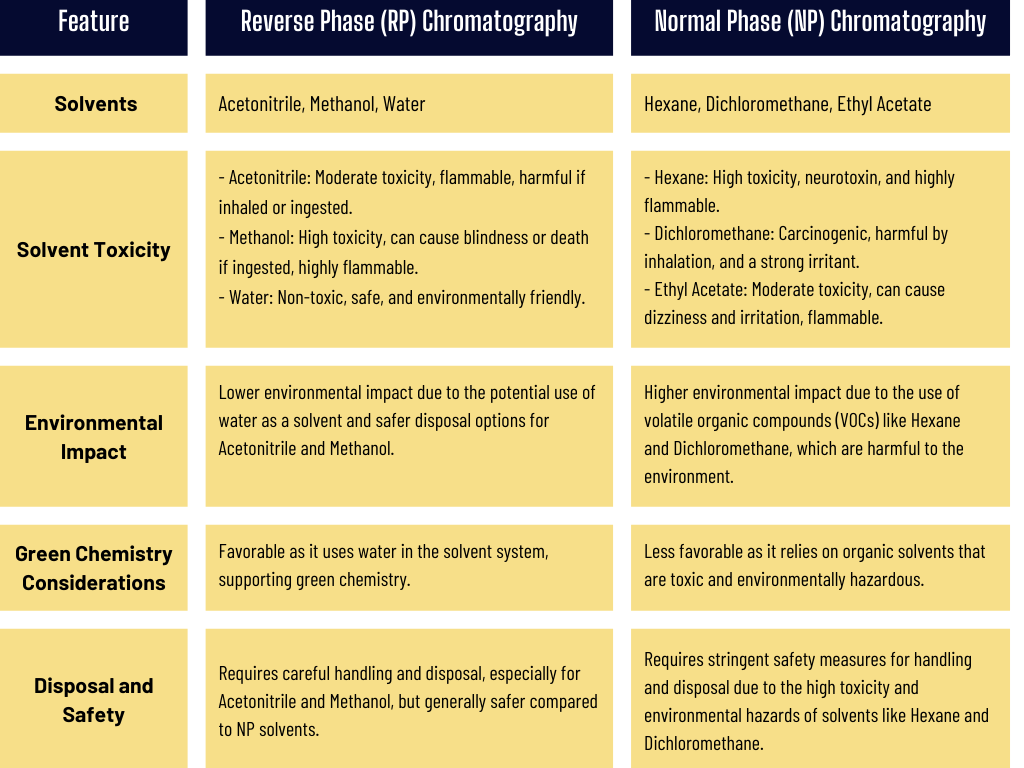
Normal phase liquid chromatography (NP-HPLC) uses a polar stationary phase, while solvents used as mobile phases in NP-HPLC are nonpolar, such as n-hexane, n-heptane, dichloromethane, dichloroethane, diethyl ether, methyl acetate, ethyl acetate, acetone, isopropanol, ethanol, or methanol.
Due to the solvents used in NP-HPLC versus RP-HPLC, it is evident that NP-HPLC involves more hazardous procedures for sample preparation and greater consumption of toxic solvents for mobile phase preparation.
SEE ALSO: RP vs NP Chromatography
Substitution of Toxic Solvents in RP-HPLC
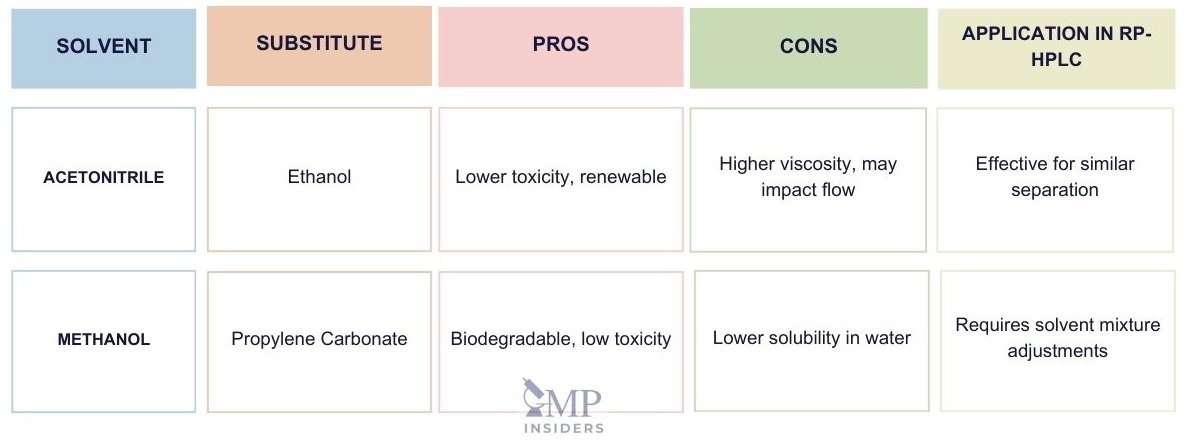
Since RP-HPLC is the most used chromatographic technique, the focus remains on maintaining the results achieved through RP-HPLC while also prioritizing the safety of quality control analysts and environmental protection. Therefore, efforts should focus on developing green analytical methods. Green analytical methods focus on substituting harmful solvents with safer alternatives that produce the same results.
Ethanol
Ethanol is a biofuel produced from renewable organic matter, such as fermenting the sugar in the starches of grains like corn and barley, or fermenting the sugar in sugar canes and sugar beets. This solvent is not toxic and is biodegradable.
Ethanol has characteristics similar to methanol and acetonitrile when used in RP-HPLC, providing comparable peak efficiency. However, ethanol has a higher viscosity, which may impact flow rates and require adjustments to column pressure and temperature. Despite these challenges, ethanol remains a promising greener solvent due to its lower toxicity and environmental impact.
Propylene Carbonate
Propylene carbonate is a polar “green” alternative solvent. It can be used as a practical alternative to acetonitrile due to its biodegradability and lower toxicity. However, propylene carbonate has low solubility in water, which can be a limitation in certain applications. To improve its solubility, it is often combined with other solvents like methanol or ethanol. While propylene carbonate offers a greener option, it requires careful optimization of solvent mixtures to ensure consistent chromatographic performance.
Acetone
Acetone and acetonitrile have similar physicochemical characteristics, such as viscosity and solubility, making acetone a potential replacement for acetonitrile in some RP-HPLC methods. Acetone is biodegradable and has low toxicity, which makes it a safer option. However, acetone has a higher UV absorption limit, up to 340 nm, which can interfere with UV detection and limit its use in certain analytical methods. Additionally, acetone’s volatility can affect retention times, necessitating careful method adjustments to achieve optimal results.
How to Get Identical Results via RP-HPLC but in a Green Way?
Chromatography offers the opportunity to reduce the usage of harmful reagents, from the preparation of the analysis to the final result.
Initially, researchers could focus on research and development of analytical methods that substitute toxic solvents with safer options. For example, ethanol can be used as a less toxic organic solvent in RP-HPLC, since its efficacy is comparable to acetonitrile and methanol. Generally, green alternatives like isopropanol, acetone, and propylene carbonate can also be used. By substituting the solvent, necessary adjustments can be made to the method to ensure satisfactory results.
Furthermore, there are columns designed for RP-HPLC that have smaller sizes. Retention time increases proportionally to the column length. This is important because the larger the column, the greater the usage of solvents for adequate washing, equilibration, and detection, which leads to increased consumption of organic solvents. Using a smaller column reduces the run time, leading to decreased solvent usage, thus reducing the generation of waste.
There are also columns with reduced particle sizes. Reducing particle size enhances separation efficiency without the need to extend run time, column length, or adjust flow rate. This will help to reduce the flow of the analytical methods, leading to reduced utilization of organic toxic solvents.
FAQ
What Are the Hazardous Solvents in RP-HPLC?
The most hazardous solvents in RP-HPLC are acetonitrile and methanol. Both are effective but pose significant risks. Acetonitrile is toxic and can cause harmful effects through inhalation or skin contact, while methanol is highly flammable and dangerous if ingested, inhaled, or absorbed. Proper handling, storage, and disposal are essential to minimize these hazards.
Which Regulations Apply to Toxic Solvents in RP-HPLC?
Using toxic solvents in RP-HPLC requires adherence to health and safety regulations, such as those from OSHA and the EPA. Regulatory agencies set exposure limits, require safety data sheets, and advise on proper disposal. Compliance with chemical safety guidelines and environmental regulations, along with thorough documentation and staff training, is crucial for safety.
How Can Laboratories Safely Use Toxic Solvents in RP-HPLC?
To ensure safe use of toxic solvents in RP-HPLC, laboratories should provide comprehensive staff training, maintain up-to-date safety protocols, and use proper PPE (gloves, goggles, lab coats). Effective ventilation and air quality monitoring are crucial. Regular maintenance of HPLC systems, proper storage, waste management, and safety audits are also key to minimizing risks.
Conclusion
While RP-HPLC remains the predominant chromatographic technique, its widespread use is associated with high consumption of organic toxic solvents and significant waste generation. Acetonitrile, favored for its low UV absorbance, low viscosity, and elution power in HPLC-UV analysis, offers notable advantages but poses health risks due to inhalation of vapors by laboratory personnel, along with methanol.
To uphold the quality of RP-HPLC results while safeguarding analysts and the environment, efforts should focus on developing new analytical methods that are environmentally friendly. Utilizing smaller columns or reducing flow rates in RP-HPLC can minimize solvent usage, contributing to these sustainability goals.
The next challenge for pharmaceutical companies should be to replace analytical methods and techniques with newer, green methods, which will highlight their concern for employees and the environment in which we all live.















