Good Manufacturing Practice (GMP) is a vital framework that ensures pharmaceuticals are consistently produced to the highest standards of quality, safety, and efficacy. More than a regulatory requirement, GMP is fundamental to protecting patient health by preventing errors that could lead to ineffective or unsafe medicines.
GMP guidelines cover every stage of manufacturing, with strict standards for quality control, process validation, and data integrity to ensure product reliability. By implementing GMP, pharmaceutical companies build transparent, trustworthy production processes that reduce risks and support public confidence in their products.
This article will explore the core principles of GMP, its evolution into current Good Manufacturing Practice (cGMP), and essential elements such as quality control, documentation, and facility cleanliness, all of which are key to producing safe, effective pharmaceuticals.
What is GMP and Why is it Important in Pharmaceutical Industry?
GMP consists of guidelines and regulations ensuring that pharmaceutical products are produced and controlled according to specific quality standards for safety and efficacy. In the pharmaceutical industry, even slight deviations from these protocols can lead to ineffective, unsafe, or contaminated medications.
GMP is designed to emphasize three primary aspects:
- Product Quality: Ensuring medications meet stringent quality standards involves measures to avoid contamination and to ensure consistency in product strength, identity, and efficacy. By following GMP, manufacturers confirm that each batch of product meets defined criteria, providing confidence in the product’s performance.
- Patient Safety: GMP is critical to preventing manufacturing errors that could result in patient harm. Rigorous testing and validation of each manufacturing process step protect against errors that might compromise safety, such as incorrect ingredient measurements, contamination, or inadequate sterilization.
- Regulatory Compliance: Adhering to GMP is mandatory for product approval and ongoing market access. Regulatory agencies across the globe enforce these standards, and non-compliance can result in severe consequences, including product recalls, fines, and facility shutdowns.
SEE ALSO: Contamination, Cross-Contamination and Mix-Ups
By following GMP, pharmaceutical companies can establish reliable processes and safeguards, providing confidence in the safety and effectiveness of their products. This is especially important because any failure in quality control or process integrity could impact public health on a large scale.
GMP vs. cGMP: Understanding the Differences
In the pharmaceutical industry, Good Manufacturing Practice (GMP) and current Good Manufacturing Practice (cGMP) both serve as critical standards to ensure product quality, safety, and consistency. While GMP provides foundational guidelines, cGMP builds on these principles by requiring manufacturers to adopt the most current practices and technologies.
Key Aspects of GMP
- Quality Control: Ensures that each step in production is controlled, documented, and conducted according to defined procedures.
- Basic Standards: GMP sets the minimum requirements for facilities, equipment, personnel, materials, and documentation to ensure consistent, safe, and effective products.
- Industry Versatility: While GMP is commonly associated with pharmaceuticals, it’s also applied to the manufacturing of cosmetics, food, medical devices, and other products that affect consumer health.
However, GMP does not explicitly require manufacturers to adopt new technologies or continuously improve their processes. This is where cGMP takes it further.
Current Good Manufacturing Practice (cGMP) builds on GMP by incorporating the requirement for continuous improvement. The “current” in cGMP implies that manufacturers must not only meet basic standards but also keep up with the latest industry practices, technologies, and regulatory expectations. The U.S. Food and Drug Administration (FDA) heavily emphasizes this concept, which is particularly critical for complex industries like pharmaceuticals.
Key Aspects of cGMP
- Continuous Improvement: cGMP requires companies to periodically assess and upgrade processes, equipment, and quality control systems to meet current standards, encouraging a proactive approach to quality.
- Risk Management: cGMP strongly emphasizes identifying, assessing, and mitigating potential risks in the production process. This involves evaluating each step for potential failures and taking preventive measures.
- Data Integrity: Accurate and tamper-proof data records are crucial in cGMP, with a strong emphasis on digital record-keeping and data security. Maintaining secure, electronic records supports traceability, transparency, and compliance.
- Advanced Monitoring: cGMP promotes the use of automated systems, digital monitoring, and real-time data analytics to enhance quality control. These systems help detect issues early, making it easier to maintain consistent quality.
RELATED: GMP vs cGMP
The 5 Ps of GMP Compliance
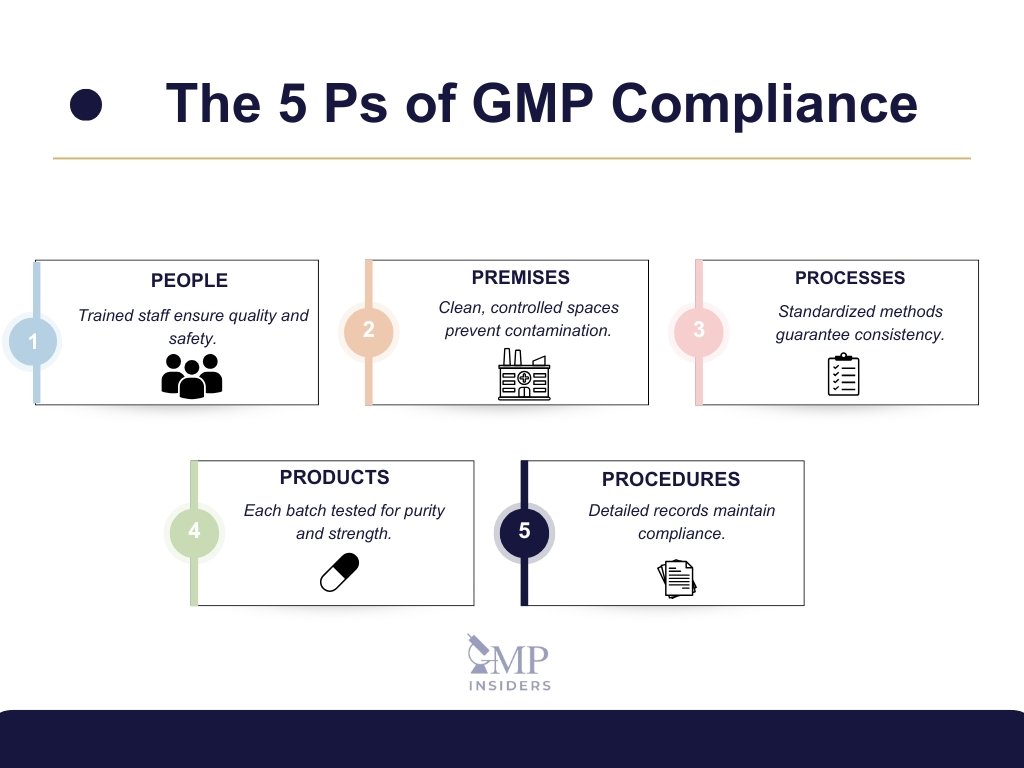
The “5 Ps” of GMP—People, Premises, Processes, Products, and Procedures—serve as a practical framework for understanding and implementing GMP requirements. Each “P” addresses a critical aspect of manufacturing, helping companies establish a comprehensive approach to quality and safety in pharmaceutical production.
1. People
GMP compliance starts with qualified, trained personnel who understand both general GMP principles and their specific roles. Ongoing training keeps staff up-to-date on industry standards and regulatory changes. Strict hygiene practices, especially in sterile environments, help prevent contamination. Skilled personnel are essential for consistent, safe, and compliant production.
2. Premises
GMP-compliant facilities are designed to minimize contamination risks, with defined zones for each production activity. Environmental controls like temperature, humidity, and air quality are closely regulated. Routine cleaning and maintenance keep the facility safe and operational. A well-organized facility supports efficient and contamination-free manufacturing.
3. Processes
Standardized and validated processes ensure consistent product quality. SOPs provide clear instructions for each task, while process validation confirms reliable results. In-process controls monitor critical parameters in real-time, helping to prevent deviations. Controlled processes are key to producing high-quality, compliant products.
4. Products
GMP focuses on ensuring every product meets strict quality, potency, and safety standards. Each batch undergoes quality control testing, with batch records providing full traceability. Proper storage and distribution conditions are maintained to preserve product integrity. These measures ensure safe, effective products reach patients.
5. Procedures (or Paperwork)
Accurate documentation provides transparency and traceability across all production stages. SOPs, batch records, and maintenance logs ensure that every step is documented and reviewable. Electronic records with audit trails enhance regulatory compliance. Reliable documentation supports inspections, quality assurance, and patient safety.
Key Components of GMP
In pharmaceutical manufacturing, GMP compliance is established through core components that uphold product quality, safety, and efficacy. Each component plays a crucial role in minimizing risk, enforcing controls, and creating a structured framework that applies to every production stage.
Quality Management System (QMS)
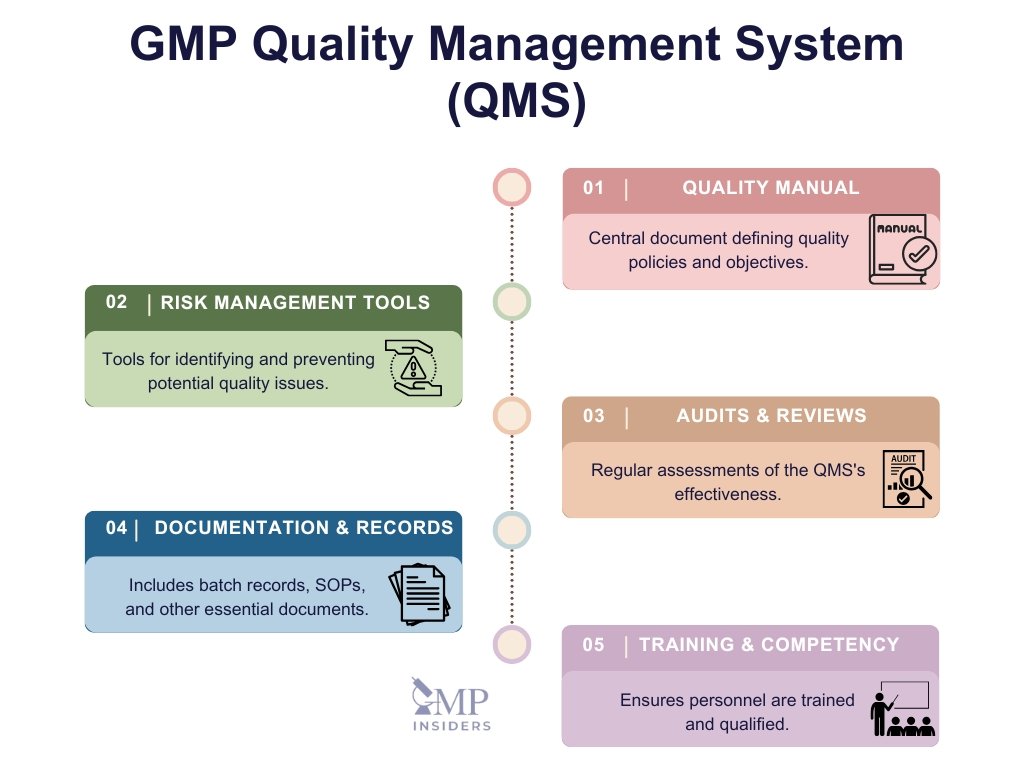
The QMS is the operational foundation for GMP compliance, integrating quality policies, processes, and responsibilities across the organization. It defines roles, sets quality objectives, and ensures consistency through documented processes. By proactively addressing quality risks, the QMS promotes a culture of compliance.
- Quality Manual: A comprehensive document that establishes organizational policies, objectives, and quality standards, aligning every department with quality goals.
- Risk Management Tools: Risk Management Tools are essential for proactively identifying, assessing, and controlling potential risks to product quality and patient safety. These tools, such as Failure Mode and Effects Analysis (FMEA) and Risk Ranking and Filtering, ensure that risk-based decision-making is embedded throughout all stages of pharmaceutical operations.
- Audits & Reviews: Regular audits and management reviews assess the effectiveness of the QMS, ensuring it meets regulatory standards and adapting as needed.
Personnel Training and Hygiene
Qualified personnel are critical for implementing GMP requirements effectively. Initial and ongoing training programs equip employees with essential GMP knowledge, while hygiene standards are essential for maintaining contamination-free environments. Routine assessments help confirm that employees remain proficient and aligned with GMP standards.
- Training Programs: Thorough training on GMP principles, contamination control, and equipment handling ensures employees understand their responsibilities and the importance of quality.
- Hygiene Standards: Use of PPE, hand-washing, and adherence to cleanroom protocols help minimize contamination risks, especially in sterile environments.
- Competency Assessments: Regular assessments, such as tests and reviews, ensure that employees continue to meet GMP standards over time.
Documentation
Accurate documentation provides traceability and accountability throughout the production lifecycle. Records like batch records and deviation reports support transparency, enabling efficient audits, investigations, and recalls when necessary. Electronic systems improve data integrity and make documentation readily accessible.
- Batch Records: Detailed logs of each production step, from raw materials to final testing, support traceability and help track issues efficiently.
- Deviation Reports: Documents deviations from SOPs and corrective actions taken, supporting root cause analysis and recurrence prevention.
- Electronic Documentation: Electronic Quality Management Systems (eQMS) help centralize and automate quality processes such as change control, deviations, CAPA, and document management. They enhance data integrity, minimize human error, improve compliance, and simplify access to records during inspections.
SEE MORE: Good Documentation Practices (GDocP)
Equipment, Facility Maintenance, and Qualification
Consistent quality depends on well-maintained, qualified equipment and facilities. GMP mandates regular calibration and qualification to ensure reliable performance. Facilities are designed to prevent contamination, with dedicated zones and environmental controls for high-sensitivity processes.
- Equipment Qualification:
- Installation Qualification (IQ): Verifies correct installation and setup of equipment.
- Operational Qualification (OQ): Confirms equipment operates as expected under standard conditions.
- Performance Qualification (PQ): Ensures equipment produces consistent, reliable results during actual production.
SEE MORE: IQ, OQ, and PQ
- Preventive Maintenance: Scheduled calibration and maintenance reduce the risk of equipment malfunctions, ensuring product quality is not compromised.
- Facility Design: Dedicated areas and environmental controls (e.g., temperature, and humidity) prevent cross-contamination and support a contamination-free workflow.
Validation
Validation in GMP environments is essential for ensuring all processes consistently meet established standards, protecting product quality and patient safety. Key types of validation include:
- Process Validation: Confirms that each production method consistently yields products meeting quality standards, ensuring process reliability.
- Cleaning Validation: Verifies that equipment cleaning procedures remove all residues, preventing cross-contamination and maintaining product purity.
SEE MORE: Cleaning Validation
- Phased Validation: Includes initial validation, ongoing monitoring, and revalidation when significant changes occur, ensuring continuous compliance.
- Validation of Analytical Methods: Ensures that laboratory methods for testing raw materials, in-process samples, and finished products are accurate, precise, and reliable, supporting consistent quality control.
- Computer System Validation (CSV): Ensures that software and digital systems used in GMP environments operate correctly, maintain data integrity, and comply with regulatory requirements, safeguarding the accuracy and security of electronic records.
SEE Also: Computer System Validation (CSV)
Deviation and Incident Management
Deviations from standard practices can occur, and a structured deviation management system ensures they are documented, investigated, and addressed to prevent a recurrence. Root cause analysis is critical for identifying the source of issues and applying Corrective and Preventive Actions (CAPA).
- Root Cause Analysis: Investigates underlying causes of deviations to guide corrective actions that prevent similar incidents.
- Trend Analysis: Regularly reviewing deviation data helps identify recurring issues and allows for proactive improvements to process control.
- Documentation: All deviations, investigations, and corrective actions are documented, providing a comprehensive record for future reference.
Related Article: Deviation Management Process in GMP
Change Control
Change control assesses the impact of modifications to processes, equipment, or materials on product quality. A structured change control process involves cross-departmental reviews, risk assessments, and documentation to ensure that quality standards are maintained despite changes.
- Change Review: Cross-functional evaluations assess the risks and impacts of proposed changes on quality.
- Documentation: Detailed records of all changes provide transparency and traceability, supporting regulatory compliance.
- Outcome: Ensures modifications are justified and controlled, maintaining GMP standards and product integrity.
Self-Inspections and Audits
Self-inspections and audits are proactive measures to identify areas of non-compliance and drive continuous improvement. Regular audits of processes, documentation, and facilities highlight areas needing attention, enabling timely corrective actions. Findings are documented, and follow-ups are tracked to ensure effective resolution.
- Internal Audits: Routine audits of processes and facilities detect compliance gaps and support quality improvement.
- Corrective Actions: Documented findings and corrective actions are tracked to closure, ensuring compliance gaps are resolved.
- Continuous Improvement: Regular audits help foster a culture of accountability and high standards throughout the organization.
RELATED: How to Prepare for a GMP Inspection
Continuous Improvement and Risk Management
Continuous improvement and risk management support sustained GMP compliance. By identifying and prioritizing risks, organizations can allocate resources effectively. Risk management tools like Failure Mode Effects Analysis (FMEA) and Hazard Analysis and Critical Control Points (HACCP) guide improvements, while feedback loops from audits and deviations drive ongoing enhancements.
- Risk Tools: FMEA and HACCP help assess risks in production processes, prioritizing areas that need improvement to maintain quality.
- Feedback Loops: Insights from audits, deviations, and complaints inform targeted improvements that strengthen processes and prevent future issues.
- Outcome: Continuous improvement ensures that high-quality standards are sustained, enhancing regulatory compliance and competitiveness.
Regulatory Framework and Global Standards
The three main agencies setting global GMP standards are the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the World Health Organization (WHO). Each organization’s approach to GMP enforcement reflects its specific regional priorities and regulatory structures, while also aligning on key principles.
U.S. Food and Drug Administration (FDA)
The FDA is the primary regulatory body for pharmaceuticals in the United States, enforcing GMP standards through its Current Good Manufacturing Practices (cGMP) outlined in Title 21 of the Code of Federal Regulations (CFR), Parts 210 and CFR Part 211. The FDA emphasizes continuous improvement, risk management, process control, and data integrity within its cGMP guidelines.
European Medicines Agency (EMA)
The EMA regulates pharmaceuticals across the European Union (EU) and ensures GMP compliance through a harmonized system, promoting consistent practices among EU member states. The EMA’s GMP guidelines are specified in EudraLex Volume 4 and enforced in coordination with national regulatory authorities.
- EudraLex Volume 4: This document outlines GMP requirements for manufacturing within the EU, including personnel qualifications, facility standards, and documentation requirements. Updated regularly, EudraLex reflects the latest advances in technology and regulatory science.
- Mutual Recognition and Collaboration: The EMA collaborates with other global regulators, such as the FDA and WHO, to harmonize GMP standards and reduce duplicate inspections. Through Mutual Recognition Agreements (MRAs), the EMA accepts GMP inspections from approved regulatory bodies in other countries, simplifying compliance for multinational companies.
- Pharmacovigilance and Safety Monitoring: The EMA manages a centralized pharmacovigilance system called EudraVigilance to monitor adverse drug reactions across the EU, enabling rapid responses to safety concerns and ensuring robust GMP enforcement.
World Health Organization (WHO)
The WHO provides GMP guidelines that are widely adopted, especially in regions lacking their own national standards. The WHO’s guidelines support global public health by promoting consistent, safe pharmaceutical production, particularly in developing countries where regulatory infrastructure may be limited.
The Future of GMP
As the pharmaceutical industry evolves, Good Manufacturing Practice (GMP) is adapting to new challenges and incorporating advanced technologies. The future of GMP will be shaped by trends in digitalization, enhanced data integrity, continuous improvement, and sustainability, which are essential to meet global regulatory and quality standards.
Integration of Pharma 5.0
Pharma 5.0 marks the next evolution in GMP, emphasizing a human-centric approach that integrates advanced technologies like AI and augmented reality (AR) to enhance decision-making and safety while prioritizing sustainability. Eco-friendly practices will become standard to minimize environmental impact.
- Data Integrity & Security: Automated systems will ensure tamper-proof records, while advanced cybersecurity measures will protect sensitive manufacturing data.
- Continuous Improvement: Emphasis on Quality by Design (QbD) and continuous manufacturing for more efficient, consistent, and reliable production.
- Regulatory Harmonization: Global standards will be streamlined, making compliance easier and ensuring access to high-quality medicines worldwide.
Enhanced Data Integrity and Security
As digital systems become more integral to GMP, data integrity and cybersecurity will be critical to maintaining trust and compliance. Automated solutions will reduce risks associated with human error and tampering.
- Automated Data Integrity Solutions will help ensure all data complies with ALCOA++ principles, making records accurate, consistent, and tamper-proof.
- Cybersecurity measures will safeguard GMP data systems from cyber threats, ensuring the reliability of sensitive manufacturing data.
Continuous Improvement and Quality by Design (QbD)
Continuous improvement remains a foundational principle of GMP, and proactive quality management will gain importance as manufacturing becomes more complex. Quality by Design (QbD) will embed quality into each product from the start.
- Continuous Manufacturing enables uninterrupted production, providing consistency and meeting high demand.
- Quality by Design (QbD) integrates quality controls into product development, minimizing risks and enhancing product reliability.
Regulatory Harmonization
In a globalized market, GMP must adapt to unified standards across regions to streamline compliance and ensure consistent product quality. Regulatory harmonization will simplify global operations and improve access to high-quality medicines.
- International Collaboration will align GMP standards, reducing regulatory complexity for multinational companies.
- Emerging Markets will benefit from uniform GMP training and compliance initiatives, supporting consistent global quality.
FAQ
How Does GMP Prevent Contamination in Pharmaceutical Manufacturing?
GMP prevents contamination through stringent guidelines on facility design, cleanroom standards, sanitation practices, and personnel hygiene. It also mandates regular cleaning, zoning of production areas, and use of personal protective equipment (PPE) to maintain a contamination-free environment.
What Is The Purpose of Process Validation in GMP?
Process validation ensures that each step in manufacturing consistently produces products that meet quality standards. By validating processes, manufacturers confirm that production methods are reliable, reducing the risk of variations that could compromise product safety or efficacy.
How Often Do Pharmaceutical Companies Undergo GMP Inspections?
GMP inspections can be scheduled or unannounced, depending on regulatory agency protocols. In general, high-risk or new facilities may be inspected more frequently, while facilities with strong compliance records might have inspections every 1-3 years.
Conclusion
Good Manufacturing Practice (GMP) is foundational to ensuring pharmaceutical products are safe, effective, and consistent. With the shift towards current Good Manufacturing Practice (cGMP), the pharmaceutical industry has embraced continuous improvement, modern technology, and proactive risk management, allowing for a more adaptive, resilient approach to quality.
As GMP evolves, innovations like real-time data monitoring, predictive analytics, and automated quality controls help manufacturers identify and prevent issues early, enhancing both efficiency and reliability.
Global harmonization of GMP standards is streamlining compliance and access to international markets, fostering consistency and trust worldwide. By staying aligned with evolving technologies and regulatory expectations, GMP ensures that pharmaceutical companies can reliably deliver high-quality medicines, safeguarding patient health and reinforcing public confidence in the products they depend on.















